Category: Parkinson's Disease: Neuroimaging
Objective: The aim was to investigate the use of R1 (relative delivery parameter) as a surrogate of blood perfusion to identify brain networks specific to Parkinson’s disease (PD).
Background: Recently, [11C]UCB-J PET has been used to measure in vivo synaptic dysregulation in PD1,2. However, most [11C]UCB-J PET studies have used binding potential or volume of distribution as the main outcome and parameters relevant to tracer delivery (K1 or R1) have been largely ignored. Given the high extraction of [11C]UCB-J from plasma, these tracer delivery parameters can be good surrogate measures of blood flow. Past work involving network analyses of blood flow3 ([15O]H2O PET, BOLD fMRI) and glucose metabolism4 ([18F]FDG) in PD have consistently identified a specific network known as PDRP. We hypothesized that PDRP network identification should be possible with R1 measure from [11C]UCB-J PET.
Method: 30 PD subjects without dementia [17F/13M, age(mean ± sd):63±8y, MDS-UPDRS Part III:30±9, disease duration:4.6±3.0y, Hoehn-Yahr:2±0] and 30 age and sex matched controls underwent [11C]UCB-J PET on the HRRT (injected activity: 580±160 MBq). R1 was estimated from SRTM2 fits to the time activity curves (ref = Centrum Semiovale, CS). The scaled subprofile model (SSM), a principal components analysis (PCA)-based regional covariance method, was used for network analysis. Briefly, R1 data from a specific set of previously published regions4 were first log-transformed and centered to minimize sources of within- and across-subject variation. Then, a PCA analysis was performed on the centered data. The second principal component, which explained about 11% variance in the data, had regional loadings that were indicative of PDRP network. The differential strength in expression of PDRP between PD subjects and HC was evaluated, along with its association with motor severity scores in PD.
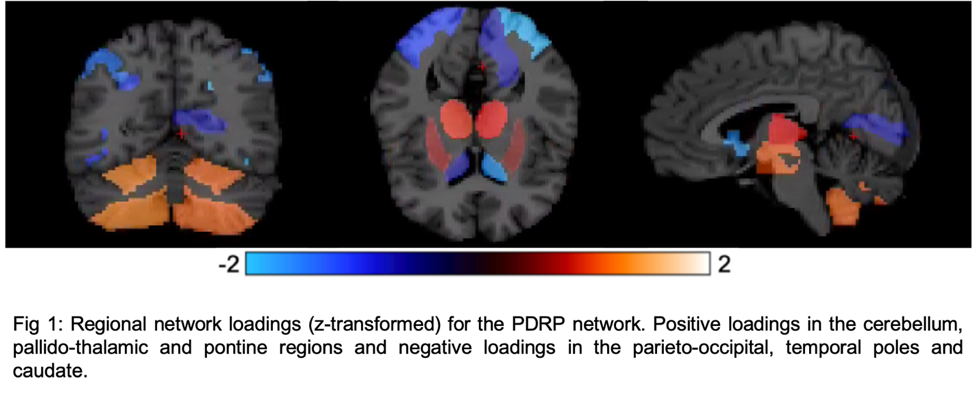
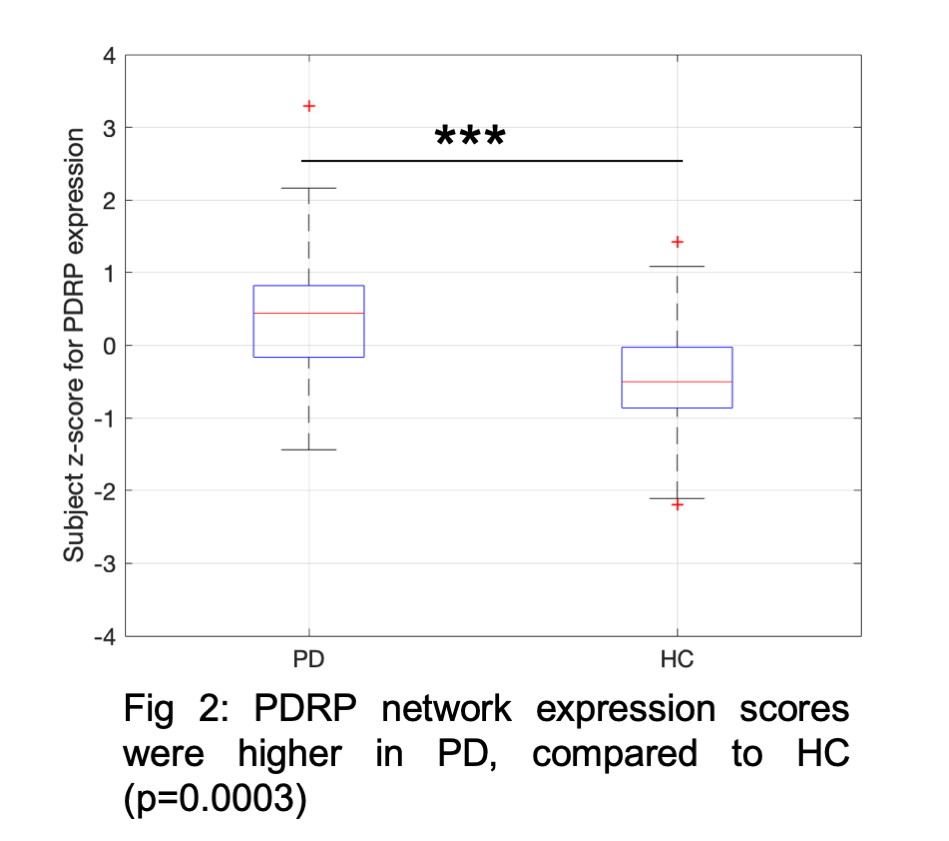
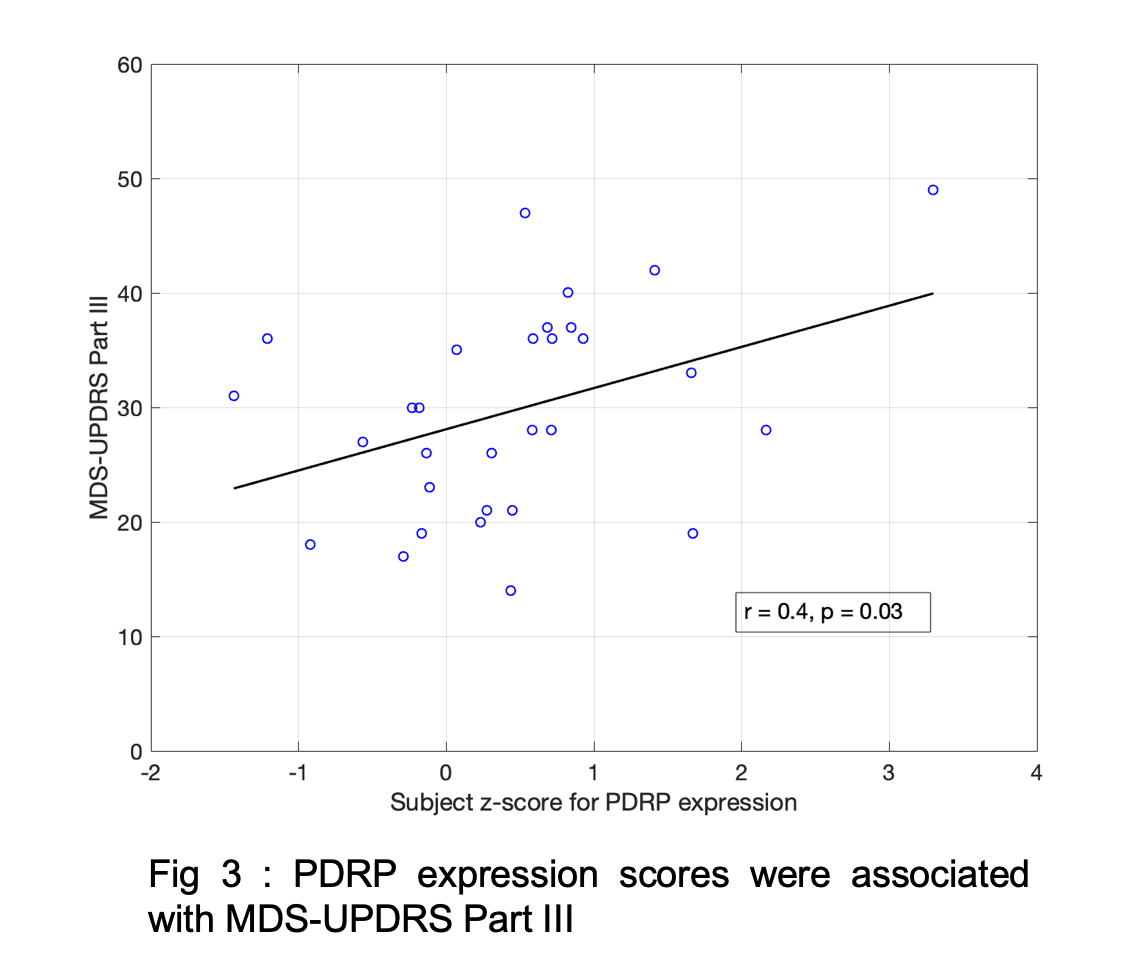
Results: Fig1 shows the identified PDRP network (positive loadings: cerebellum, pallidothalamic and pontine regions, negative loadings: parieto-occipital, inferior parietal and temporal poles). Subject z-scores, representing the strength of this network, were higher in PD (p=0.0003,Fig2). Finally, subject z-scores were also significantly associated with MDS-UPDRS Part III in PD subjects (r=0.4,p=0.03,Fig3).
Conclusion: Early part of [11C]UCB-J scan is sensitive to network-based covariance patterns in PD that are usually defined with blood flow measures.
References: 1. Matuskey, D. et al. (2020). Ann. Neurol., 87(3), 329-38
2. Delva, A. et al. (2020). Mov. Disord., 35(11), 1977-86
3. Ma, Y. et al. (2007). Mol Imaging Biol., 9, 223-233
4. Eidelberg, D. et al. (1994). JCBFM, 14(5), 783-801
To cite this abstract in AMA style:
P. Honhar, S. Tinaz, S. Holmes, M. Dias, M. Naganawa, J. Gallezot, S. Henry, R. Comley, N. Nabulsi, Y. Huang, A. Hillmer, R. Carson, S. Finnema, D. Matuskey. Network analysis of brain perfusion in [11C]UCB-J PET: Parkinson’s related motor pattern (PDRP) [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/network-analysis-of-brain-perfusion-in-11cucb-j-pet-parkinsons-related-motor-pattern-pdrp/. Accessed December 23, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/network-analysis-of-brain-perfusion-in-11cucb-j-pet-parkinsons-related-motor-pattern-pdrp/