Session Information
Date: Monday, October 8, 2018
Session Title: Rating Scales
Session Time: 1:15pm-2:45pm
Location: Hall 3FG
Objective: To find the MCID of the UPDRS-III subscale in early PD subjects, when started on a new dopaminergic drug.
Background: The concept of Minimally Clinical Important Difference (MCID) intends to establish the smallest difference in a rating scale that is perceived by patients as beneficial or harmful, and which would lead the clinician to consider a meaningful change in treatment. The UPDRS-III, or its newer version MDS-UPDRS-III, are the most used scales for clinical evaluation and follow up of motor signs PD. For that reason, it is pertinent to use this subscale to define the cutoff, for improvement or worsening when a new treatment is initiated in PD. This approach has been investigated before, using data of clinical trials [1]. We aimed to validate this method using a naturalistic approach.
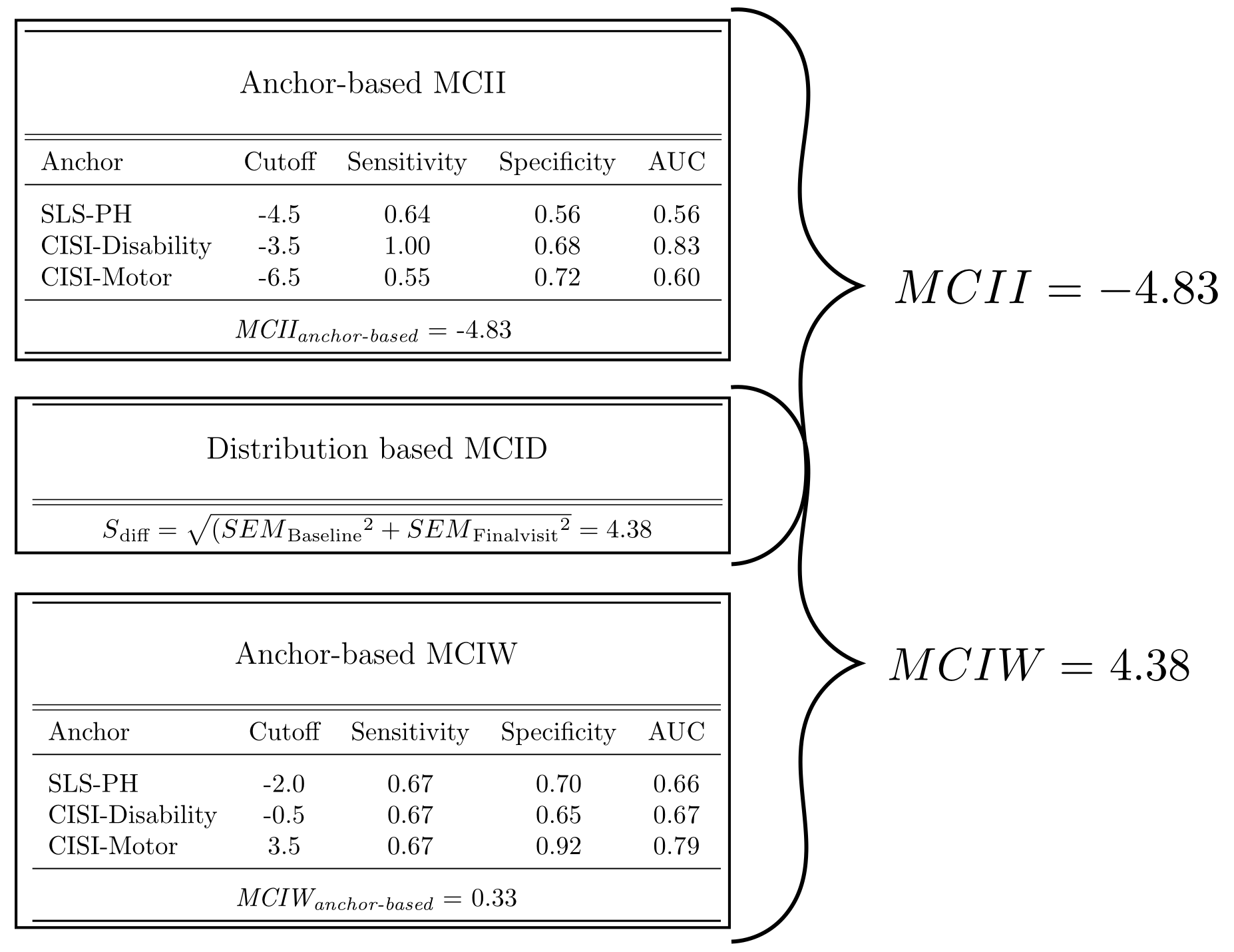
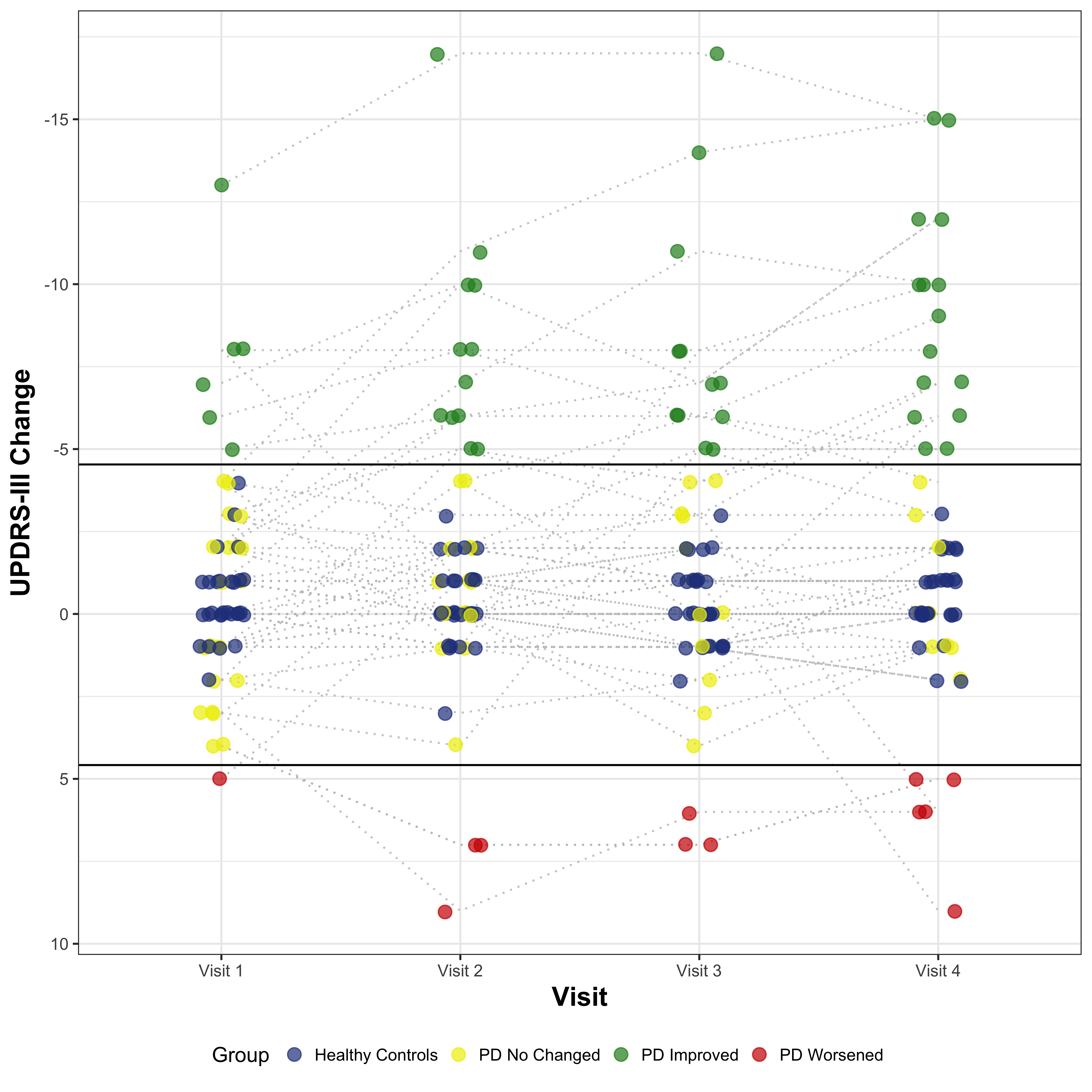
Methods: We followed for 6 months a cohort of 30 recently diagnosed PD subjects who were starting a dopaminergic treatment. For calculating the MCID, we employed a dual approach, combining distribution- and anchor-based methods [2]. Regarding the anchor-based, we used three different anchors on perceived physical health: the Physical Health item of the SLS-6, the Disability and the Motor Signs items of the CISI-PD. We defined as improved the subjects with a change of at least 1 point in the positive sense compared to baseline and as worsened a change of at least 1 point in the negative sense. Based on that definition we performed a ROC Curve Analysis of the ∆UPDRS-III for each anchor for classification of improvement and worsening to find the best cutoff and then triangulated them averaging the results. For the distribution-based method, we employed the Standard error of the difference (Sdiff) to define the minimum change not attributable to measurement error. Finally, we combined this 2 methods, selecting as final threshold the more stringent cutoff between the two.
Results: The anchor-based MCID for Improvement (MCII) and MCID for Worsening (MCIW) thresholds (both based on the triangulation of the 3 cutoffs) were respectively -4.83 and +0.33 point change of UPDRS-III from baseline. The Sdiff was 4.38 point change of UPDRS-III from baseline. Combining both approaches we finally had the following threshold: – MCII = -4.83 – MCIW = +4.38 Considering that the UPDRS-III is a discrete scale, in both cases the minimal change equal or superior to the obtained values corresponded to a 5-point change from the baseline.
Conclusions: A 5-point change from baseline was found to be the MCID in early PD population, when introducing a new dopaminergic drug.
References: 1 Schrag A, Sampaio C, Counsell N, et al. Minimal clinically important change on the unified Parkinson’s disease rating scale. Mov Disord 2006;21:1200–7. 2 Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008;61:102–9.
To cite this abstract in AMA style:
M. Matarazzo, P. Martínez Martín, J.C. Martínez-Ávila, A. Gómez de-la-Cámara, L. Giancardo, T. Arroyo Gallego, P. Montero Escribano, V. Puertas-Martín, I. Obeso, I. Butterworth, C.S. Mendoza, M.J. Catalán, J.A. Molina-Arjona, F. Bermejo-Pareja, J.C. Martínez-Castrillo, L. López-Manzanares, A. Alonso-Canovas, J. Herreros-Rodríguez, M. Gray, Á. Sánchez-Ferro. Naturalistic-based UPDRS-III Minimal Clinically Important Difference (MCID) in early PD [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/naturalistic-based-updrs-iii-minimal-clinically-important-difference-mcid-in-early-pd/. Accessed January 1, 2026.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/naturalistic-based-updrs-iii-minimal-clinically-important-difference-mcid-in-early-pd/