Session Information
Date: Wednesday, June 22, 2016
Session Title: Ataxia
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: The present study was aimed to assess the progression of saccade involvement in SCA2 patients, identify its main determinants and evaluate its usefulness as outcome measures in clinical trials.
Background: Saccadic eye movement abnormalities are frequent features of Spinocerebellar Ataxia type 2 (SCA2) patients [1,2]. Nevertheless the natural history of these alterations is unknown, limiting its helpfulness as outcome measure for clinical trials.
Methods: We performed a prospective 5-year follow-up study of saccadic abnormalities in 30 SCA2 patients and their sex-and age-matched healthy controls. All subjects were evaluated a total of four times by clinical and electrooculographical assessments of horizontal saccades and by Scale for Assessing and Rating of Ataxia (SARA).
Results: SCA2 patients showed significant decreases in saccade peak velocity and saccade accuracy, and increases of saccadic latency during the follow-up period. 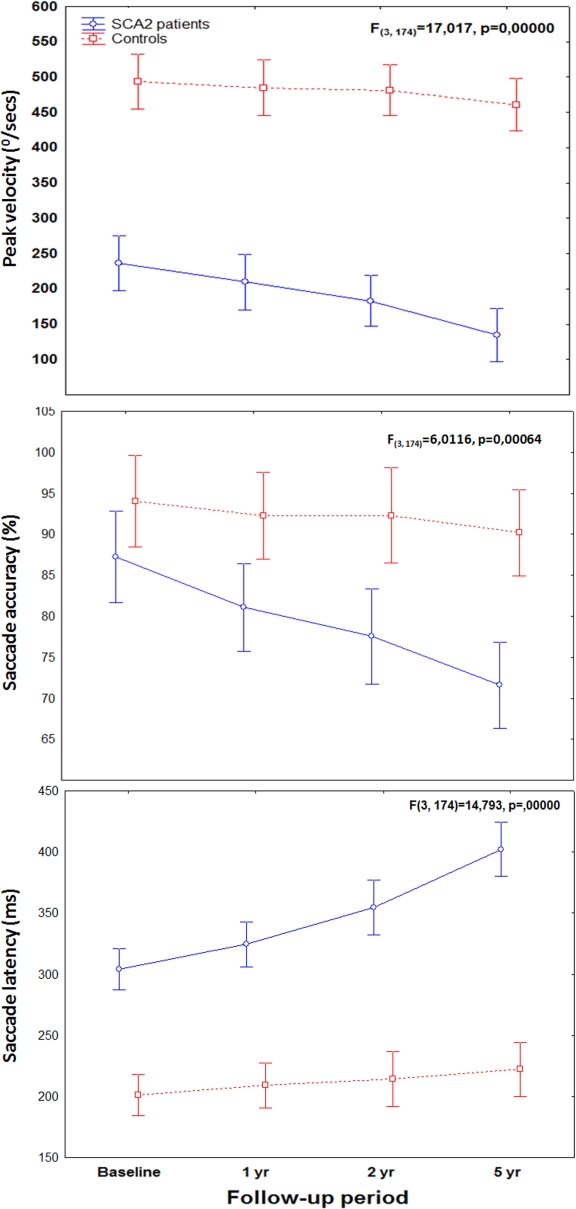 Annual progression rates were significantly higher in patients compared to controls. Faster progression rates of saccade slowing were associated with higher trinucleotide CAG-repeat expansions. Sample size estimates for two-arm trials would require 19 patients per group to detect a 50% reduction in disease progression using saccade peak velocity as outcome variable, but 44 and 124 patients using saccade latency and accuracy, respectively (power, 80%; alpha=0.05). The same analysis applied to the SARA score in our cohort revealed an estimation of 70 subjects per group.
Annual progression rates were significantly higher in patients compared to controls. Faster progression rates of saccade slowing were associated with higher trinucleotide CAG-repeat expansions. Sample size estimates for two-arm trials would require 19 patients per group to detect a 50% reduction in disease progression using saccade peak velocity as outcome variable, but 44 and 124 patients using saccade latency and accuracy, respectively (power, 80%; alpha=0.05). The same analysis applied to the SARA score in our cohort revealed an estimation of 70 subjects per group.
Conclusions: Electrooculographical measures of saccade changes are useful for the objective quantification of disease course in SCA2. The progression rate of saccade slowing is highly influenced by the CAG-repeat expansion size, providing novel insight into the cumulative polyglutamine neurotoxicity, and supporting the usefulness of saccade peak velocity as a sensitive biomarker during the natural history of the disease, and as suitable outcome measure for future therapeutic trials, with high sensitivity in comparison to clinical scales. References: 1.Velazquez-Perez L, Seifried C, Santos-Falcon N, et al. Saccade velocity is controlled by polyglutamine size in spinocerebellar ataxia 2. Ann Neurol 2004;56:444-447. 2.MacAskill MR, Anderson TJ. Eye movements in neurodegenerative diseases. Curr Opin Neurol. 2016; 29(1):61-68.
To cite this abstract in AMA style:
R. Rodríguez-Labrada, L. Velázquez-Pérez, G. Auburger, U. Ziemann, Y. Vazquez-Mojena, N. Canales-Ochoa. Natural history of saccadic abnormalities in spinocerebellar ataxia 2: Implications to designing future clinical trials [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/natural-history-of-saccadic-abnormalities-in-spinocerebellar-ataxia-2-implications-to-designing-future-clinical-trials/. Accessed April 26, 2025.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/natural-history-of-saccadic-abnormalities-in-spinocerebellar-ataxia-2-implications-to-designing-future-clinical-trials/
