Category: Parkinson's Disease: Non-Motor Symptoms
Objective: The aim of this study is to develop and validate a prediction model of AOHPL to facilitate physicians in identifying patients at higher probability of developing AOHPL.
Background: Levodopa could induce orthostatic hypotension (OH) in Parkinson’s disease (PD) patients. It reduced quality of life, and aggravated burden of disease. Accurate prediction of acute OH post levodopa (AOHPL) is important for not only rational, safe, and effective drug use in PD patients but also for data-based treatment decision-making.
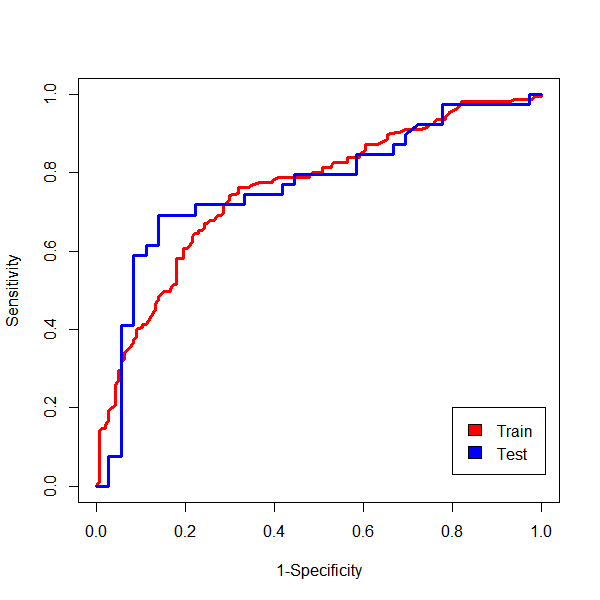
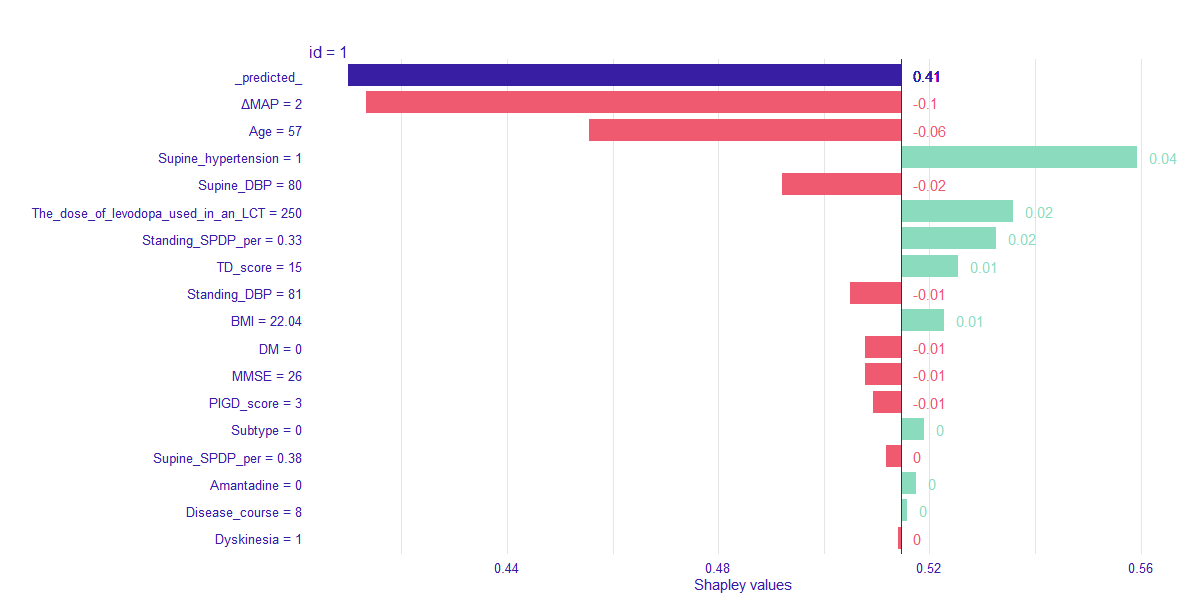
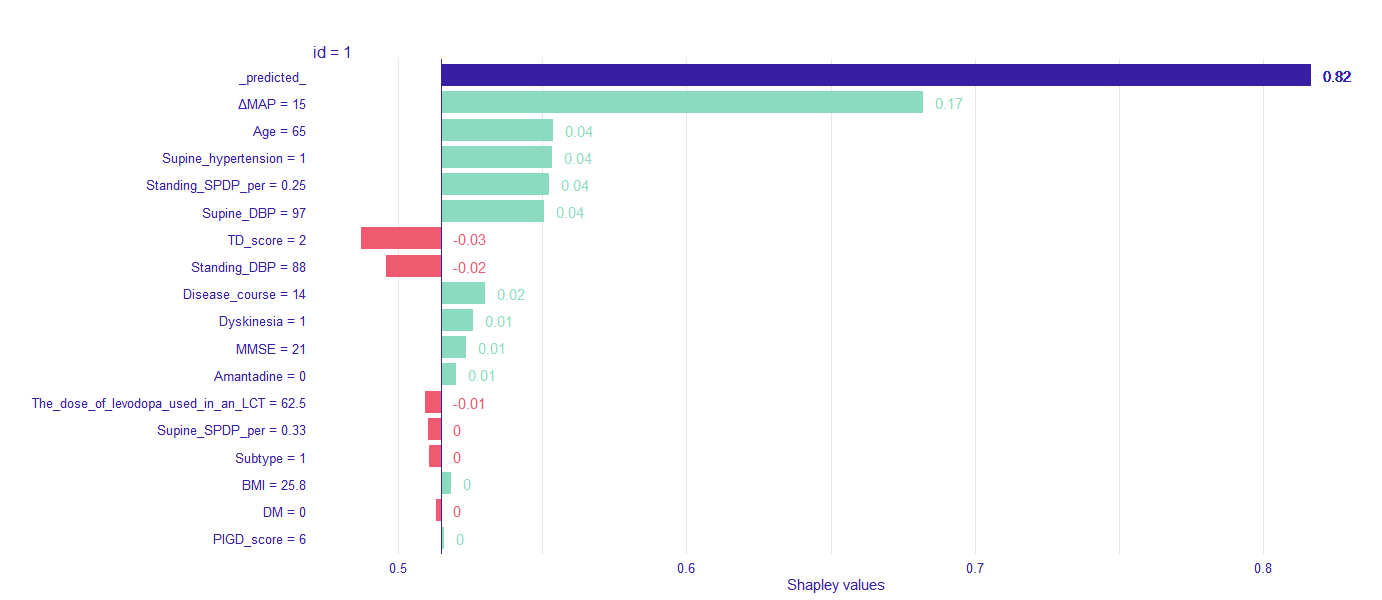
Method: We examined the probability of AOHPL among 497 PD inpatients who underwent levodopa challenge test (LCT) and Supine-to-standing test (STS) during LCT. Patients were classified into two groups, OH occurred after taken levodopa during LCT were involved in AOHPL cohort, otherwise in NON-AOHPL group. The whole dataset was randomly split into training (80%) and independent test data (20%). Logistic Regression (LR), Support Vector Machine (SVM) and Random Forest (RF) were trained on the training set for prediction of AOHPL. The leave-one-out cross validation (LOOCV) performance of these models were compared. Independent test data provided an evaluation of the final predictive model which had the best LOOCV performance. Shapley additive explanations (SHAP) values were used to disclose how variables explain the specific prediction on a given observation and associated prediction on independent test data.
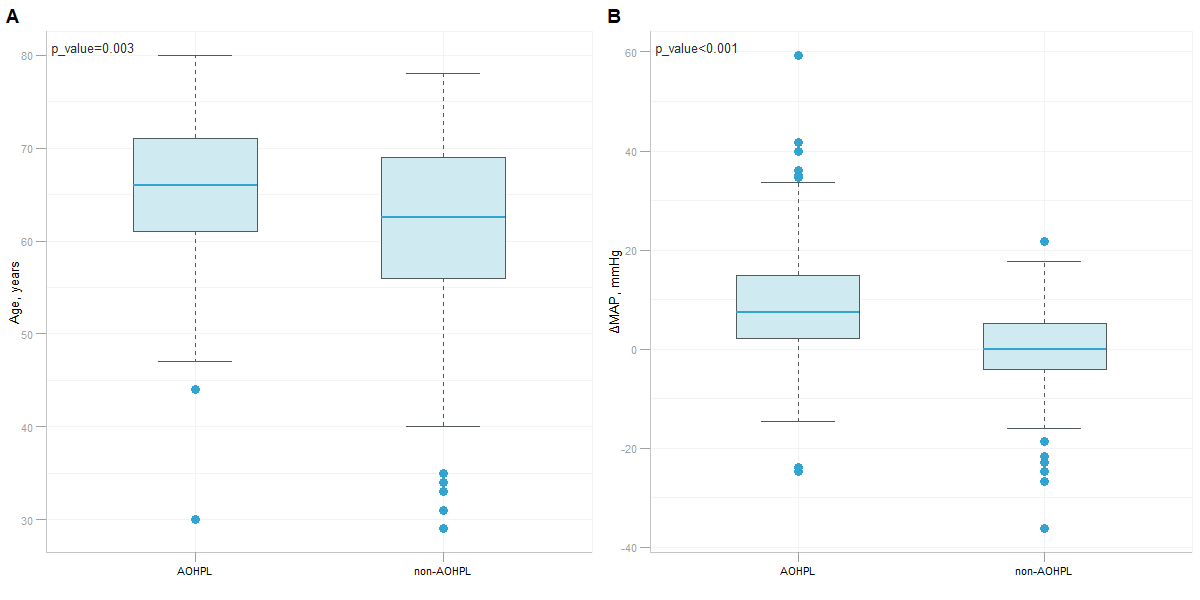
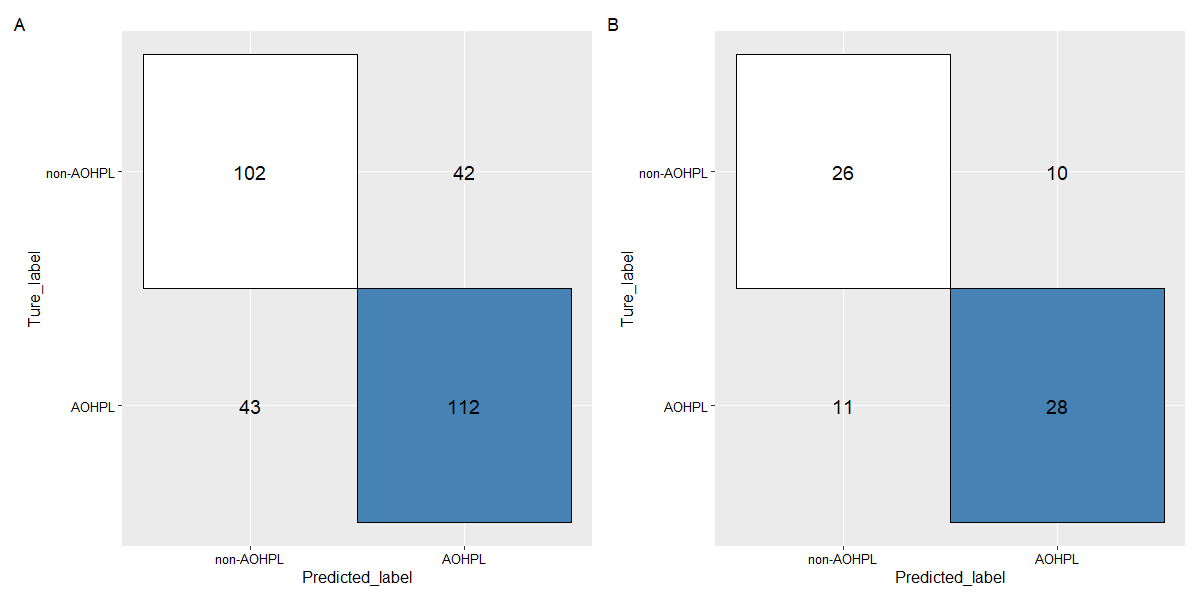
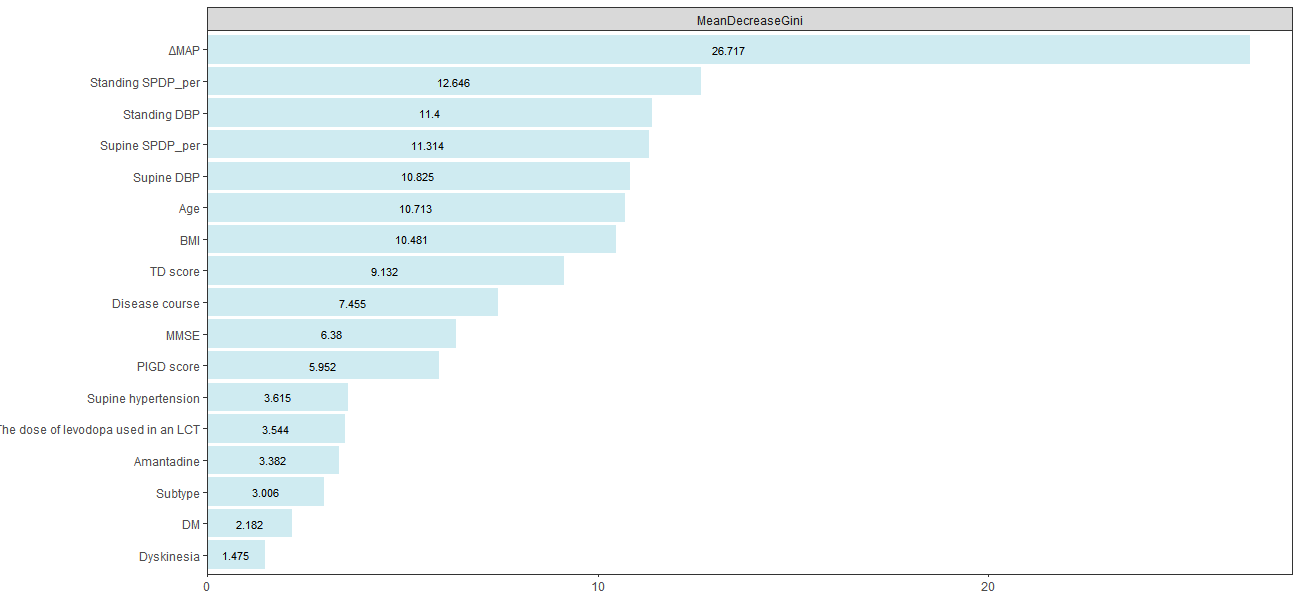
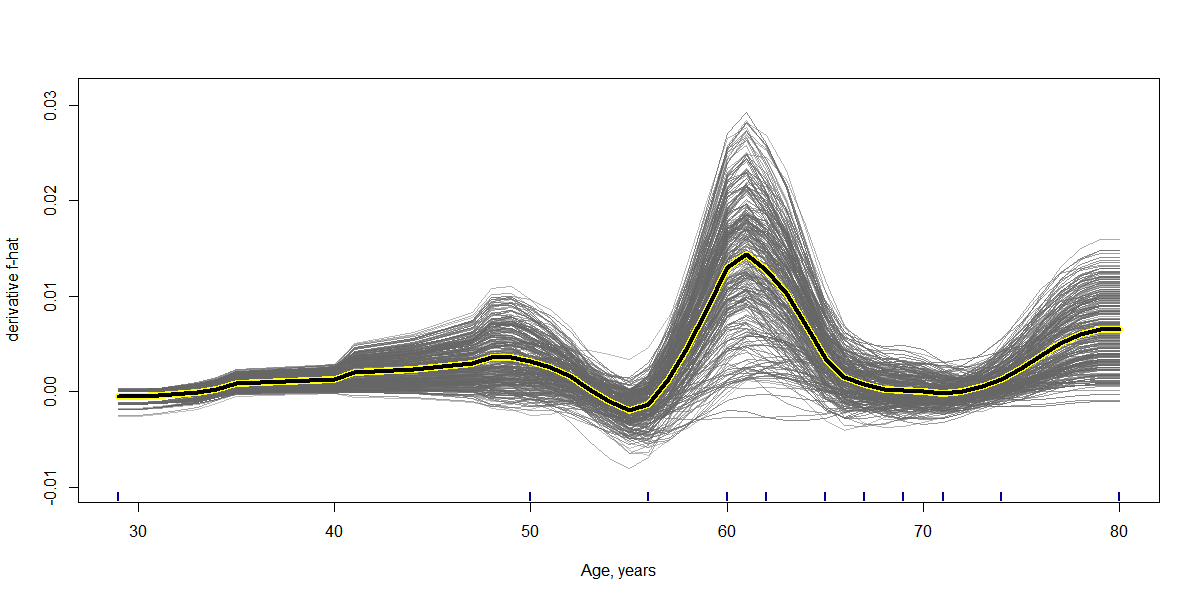
Results: RF was selected as our final predictive model as its LOOCV performance [Accuracy 71.6%, Sensitivity 72.3%, Specificity 70.8%] outperformed other models [Accuracy, Sensitivity, Specificity are 69.2%, 69.7%, 68.8% for LR, and 70.6%, 71.6%, 69.4% for SVM, respectively]. 17 variables were included in the constructive RF model. Mean artery pressure drop (ΔMAP) was the most important feature in this predictive model. For independent test data, we achieved a prediction accuracy of 72%. ΔMAP, age and supine hypertension were the top three variables which explain most to the prediction across all individual observation on the independent test data.
Conclusion: A random forest classifier model can be used to predict PD patients to develop AOHPL or not through a routine data-driven approach. This validated classifier could help clinicians to recognize high risk of AOHPL early, and to provide suggestion for treatment decision-making preventing OH, improving the quality of life in PD patients.
References: 1. Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM. Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17(10):724-729.
2. Dommershuijsen LJ, Heshmatollah A, Mattace Raso FUS, Koudstaal PJ, Ikram MA, Ikram MK. Orthostatic Hypotension: A Prodromal Marker of Parkinson’s Disease? Mov Disord. 2021;36(1):164-170.
3. Palma JA, Kaufmann H. Orthostatic Hypotension in Parkinson Disease. Clin Geriatr Med. 2020;36(1):53-67.
4. Wieling W, Kaufmann H, Claydon VE, et al. Diagnosis and treatment of orthostatic hypotension. Lancet Neurol. 2022;21(8):735-746.
5. Hiorth YH, Pedersen KF, Dalen I, Tysnes OB, Alves G. Orthostatic hypotension in Parkinson disease: A 7-year prospective population-based study. Neurology. 2019;93(16):e1526-e1534.
6. Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol. 2016;15(9):954-966.
7. McDonald C, Newton JL, Burn DJ. Orthostatic hypotension and cognitive impairment in Parkinson’s disease: Causation or association? Mov Disord. 2016;31(7):937-946.
8. De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL, Warner TT. Association of Autonomic Dysfunction With Disease Progression and Survival in Parkinson Disease. JAMA Neurol. 2017;74(8):970-976.
9. Zhu S, Li H, Xu X, et al. The Pathogenesis and Treatment of Cardiovascular Autonomic Dysfunction in Parkinson’s Disease: What We Know and Where to Go. Aging Dis. 2021;12(7):1675-1692.
10. Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord. 2015;30(5):639-645.
11. Sanchez-Ferro A, Benito-Leon J, Gomez-Esteban JC. The management of orthostatic hypotension in Parkinson’s disease. Front Neurol. 2013;4:64.
12. Kujawa K, Leurgans S, Raman R, Blasucci L, Goetz CG. Acute orthostatic hypotension when starting dopamine agonists in Parkinson’s disease. Arch Neurol. 2000;57(10):1461-1463.
13. Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670-1683.
14. Mehagnoul-Schipper DJ, Boerman RH, Hoefnagels WH, Jansen RW. Effect of levodopa on orthostatic and postprandial hypotension in elderly Parkinsonian patients. J Gerontol A Biol Sci Med Sci. 2001;56(12):M749-755.
15. Goldstein DS. Dysautonomia in Parkinson’s disease: neurocardiological abnormalities. Lancet Neurol. 2003;2(11):669-676.
16. Noack C, Schroeder C, Heusser K, Lipp A. Cardiovascular effects of levodopa in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(8):815-818.
17. Asahina M, Vichayanrat E, Low DA, Iodice V, Mathias CJ. Autonomic dysfunction in parkinsonian disorders: assessment and pathophysiology. J Neurol Neurosurg Psychiatry. 2013;84(6):674-680.
18. Pathak A, Senard JM. Pharmacology of orthostatic hypotension in Parkinson’s disease: from pathophysiology to management. Expert Rev Cardiovasc Ther. 2004;2(3):393-403.
19. Jordan J, Fanciulli A, Tank J, et al. Management of supine hypertension in patients with neurogenic orthostatic hypotension: scientific statement of the American Autonomic Society, European Federation of Autonomic Societies, and the European Society of Hypertension. J Hypertens. 2019;37(8):1541-1546.
20. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591-1601.
21. Cutsforth-Gregory JK, Low PA. Neurogenic Orthostatic Hypotension in Parkinson Disease: A Primer. Neurol Ther. 2019;8(2):307-324.
22. Feng T, Li W, Lu L, et al. Acute stepwise challenge test with levodopa in treated patients with parkinsonism. Parkinsonism Relat Disord. 2009;15(5):354-358.
23. Albanese A, Bonuccelli U, Brefel C, et al. Consensus statement on the role of acute dopaminergic challenge in Parkinson’s disease. Mov Disord. 2001;16(2):197-201.
24. Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord. 2013;28(5):668-670.
25. Juraschek SP, Daya N, Rawlings AM, et al. Association of History of Dizziness and Long-term Adverse Outcomes With Early vs Later Orthostatic Hypotension Assessment Times in Middle-aged Adults. JAMA Intern Med. 2017;177(9):1316-1323.
26. Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264(8):1567-1582.
27. Chan YH. Biostatistics 104: correlational analysis. Singapore Med J. 2003;44(12):614-619.
28. Goldstein A, Kapelner A, Bleich J, Pitkin E. Peeking Inside the Black Box: Visualizing Statistical Learning With Plots of Individual Conditional Expectation. Journal of Computational and Graphical Statistics. 2015;24(1):44-65.
29. Lundberg SM, Lee S-I. A Unified Approach to Interpreting Model Predictions. Paper presented at: NIPS2017.
30. Perez-Lloret S, Rey MV, Fabre N, et al. Factors related to orthostatic hypotension in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(5):501-505.
31. Fanciulli A, Campese N, Goebel G, et al. Association of transient orthostatic hypotension with falls and syncope in patients with Parkinson disease. Neurology. 2020;95(21):e2854-e2865.
32. Umehara T, Nakahara A, Matsuno H, Toyoda C, Oka H. Predictors of postprandial hypotension in elderly patients with de novo Parkinson’s disease. J Neural Transm (Vienna). 2016;123(11):1331-1339.
33. Fanciulli A, Jordan J, Biaggioni I, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS) : Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res. 2018;28(4):355-362.
34. Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med. 2008;358(6):615-624.
35. Sharabi Y, Goldstein DS. Mechanisms of orthostatic hypotension and supine hypertension in Parkinson disease. J Neurol Sci. 2011;310(1-2):123-128.
36. Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498-2508.
37. Saranza G, Lang AE. Levodopa challenge test: indications, protocol, and guide. J Neurol. 2021;268(9):3135-3143.
To cite this abstract in AMA style:
Z. Liu, SN. Lin, ZL. Chen, Y. Ling, T. Feng. Machine Learning Model for Prediction of acute orthostatic hypotension after levodopa administration [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/machine-learning-model-for-prediction-of-acute-orthostatic-hypotension-after-levodopa-administration/. Accessed January 1, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/machine-learning-model-for-prediction-of-acute-orthostatic-hypotension-after-levodopa-administration/