Session Information
Date: Thursday, June 8, 2017
Session Title: Parkinson's Disease: Neuroimaging And Neurophysiology
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: To map regional differences in rates of change in diffusion tensor imaging (DTI)-based measures of brain white matter microstructural integrity between Parkinson (PD) patients and age-matched controls over 18 months.
Background: Post-mortem studies suggest that in Parkinson’s disease, Lewy bodies accumulate first in the olfactory bulb, followed sequentially by caudal brainstem, substantia nigra, limbic cortex, and neocortex (Braak, 2003). Studies of a variety of traumatic and degenerative brain diseases suggest that DTI is sensitive, if not specific, to early disease-related white matter change. We hypothesized that the rate of microstructural change in PD would exceed that of normal aging, and would reflect it’s theorized topographic progression.
Methods: Seventy-two (29 PD, 43 control) participants completed a brain MRI that included 40-direction DTI, T1, and fluid sensitive inversion recovery (FLAIR) sequences at baseline and 18 month follow-up. DTI series were corrected for motion and eddy current distortions, followed by tensor fitting in Camino (UCL). Iterative tensor-based registration (Keihaninejad et al., 2013) was completed to yield fractional anisotropy (FA), mean (MD), and radial diffusivity (RD) maps in population space. White matter hyperintensity (WMH) maps derived from FLAIR and T1 images using the lesion segmentation toolbox for SPM12 (fil.ion.ucl.ac.uk) were also coregistered to population space. Regression models were constructed to evaluate the effect of group (PD versus control) on the degree of FA, MD, and RD change in the follow-up image relative to the baseline image using the Biological Parametric Mapping toolbox for SPM5. Covariates included the WMH, age, gender, education, and number of months between visits. Monte Carlo simulation was used to establish an a priori cluster-corrected threshold of .05 at 85 voxels of P(voxel)<.005.
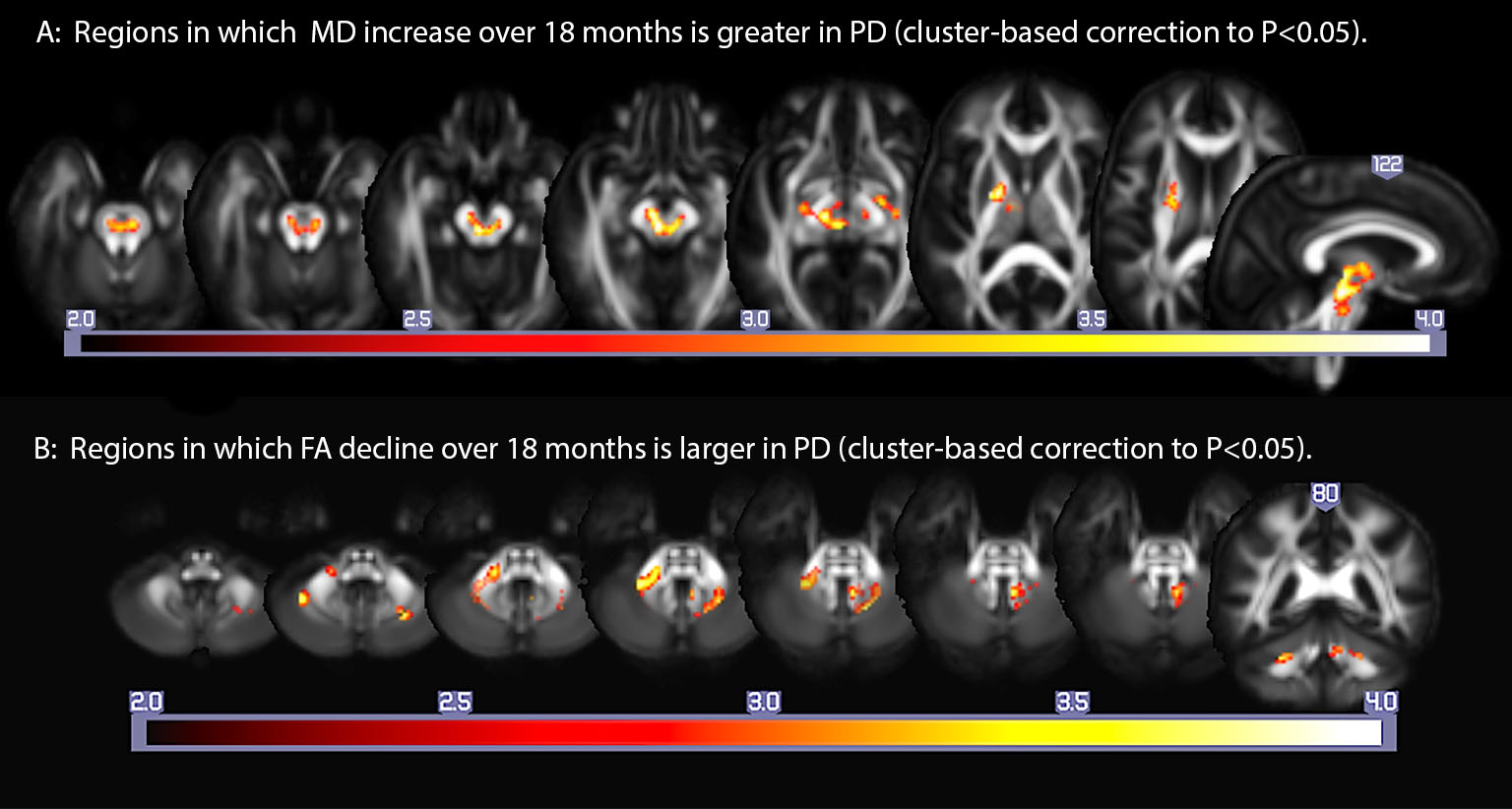
Results: Relative to baseline, the PD group showed greater increases in MD and RD within the pontine and midbrain tegmentum, as well as portions of deep subcortical white matter and internal capsule, and a greater decrease in FA in deep cerebellar white matter and middle cerebellar peduncle (Figure).
Conclusions: PD is associated with a greater longitudinal decline in white matter integrity in the brainstem, caudal subcortical white matter, and cerebellum, than is normal aging. This pattern is commensurate with the Braak hypothesis, with the exception of cerebellar changes.
References:
- Braak H, De Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging 2003; 24: 197-210.
- Keihaninejad S, Zhang H, Ryan N, et al. An unbiased longitudinal analysis framework for tracking white matter changes using diffusion tensor imaging with application to Alzheimer disease. Neuroimage 2013; 72: 153-63.
To cite this abstract in AMA style:
C. Gallagher, V. Pozorski, J. Oh, F. Theisen, N. Adluru, A. Alexander. Longitudinal white matter microstructural change in Parkinson’s disease [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/longitudinal-white-matter-microstructural-change-in-parkinsons-disease/. Accessed April 18, 2025.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/longitudinal-white-matter-microstructural-change-in-parkinsons-disease/