Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate long-term safety and efficacy of foslevodopa/foscarbidopa (LDp/CDp) among people with advanced Parkinson’s disease (aPD).
Background: As aPD progresses, erratic gastric emptying and pulsatile dopaminergic stimulation contribute to unpredictable levodopa (LD) response and motor fluctuations. LDp/CDp is a soluble formulation of LD/carbidopa (CD) prodrugs administered as a 24-hour/day continuous subcutaneous infusion. In a 12-week randomized phase 3 study (NCT04380142), LDp/CDp demonstrated a favorable risk/benefit profile compared with oral immediate-release LD/CD [1]. Patients (pts) who completed that study were eligible to enroll in a 96‑week open‑label extension (OLE; NCT04750226). We report interim results for pts who received LDp/CDp in the parent study and continued receiving LDp/CDp in the OLE; data from pts receiving oral LD/CD in the parent study will be presented separately.
Method: On entering the parent study, pts were aged ≥30 years with LD-responsive, inadequately controlled (≥2.5 hours “Off” time/day) idiopathic PD. LDp/CDp infusions are individually optimized at approximately 600–4250 mg LD equivalents/24 hours. The primary endpoint is safety and tolerability. Data are presented through week 48 of the OLE; >1 year LDp/CDp duration including the 12‑week parent study treatment period (interim cutoff, September 14, 2022). Baseline values for the OLE were collected at entry into the OLE.
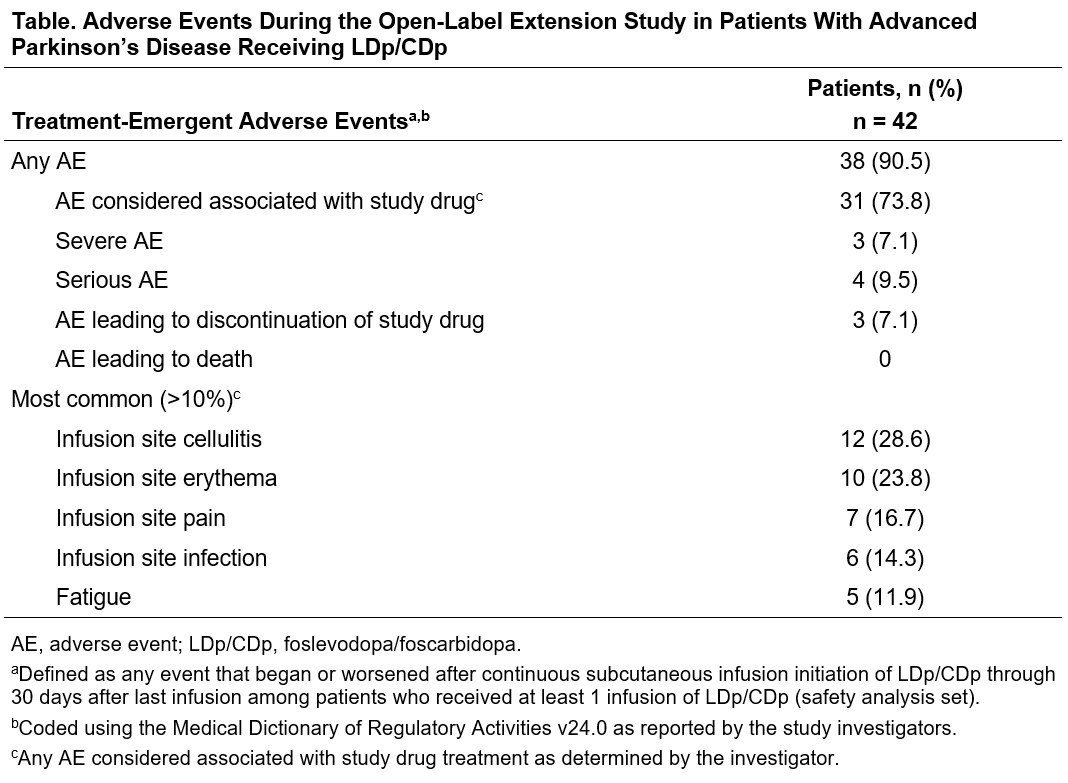
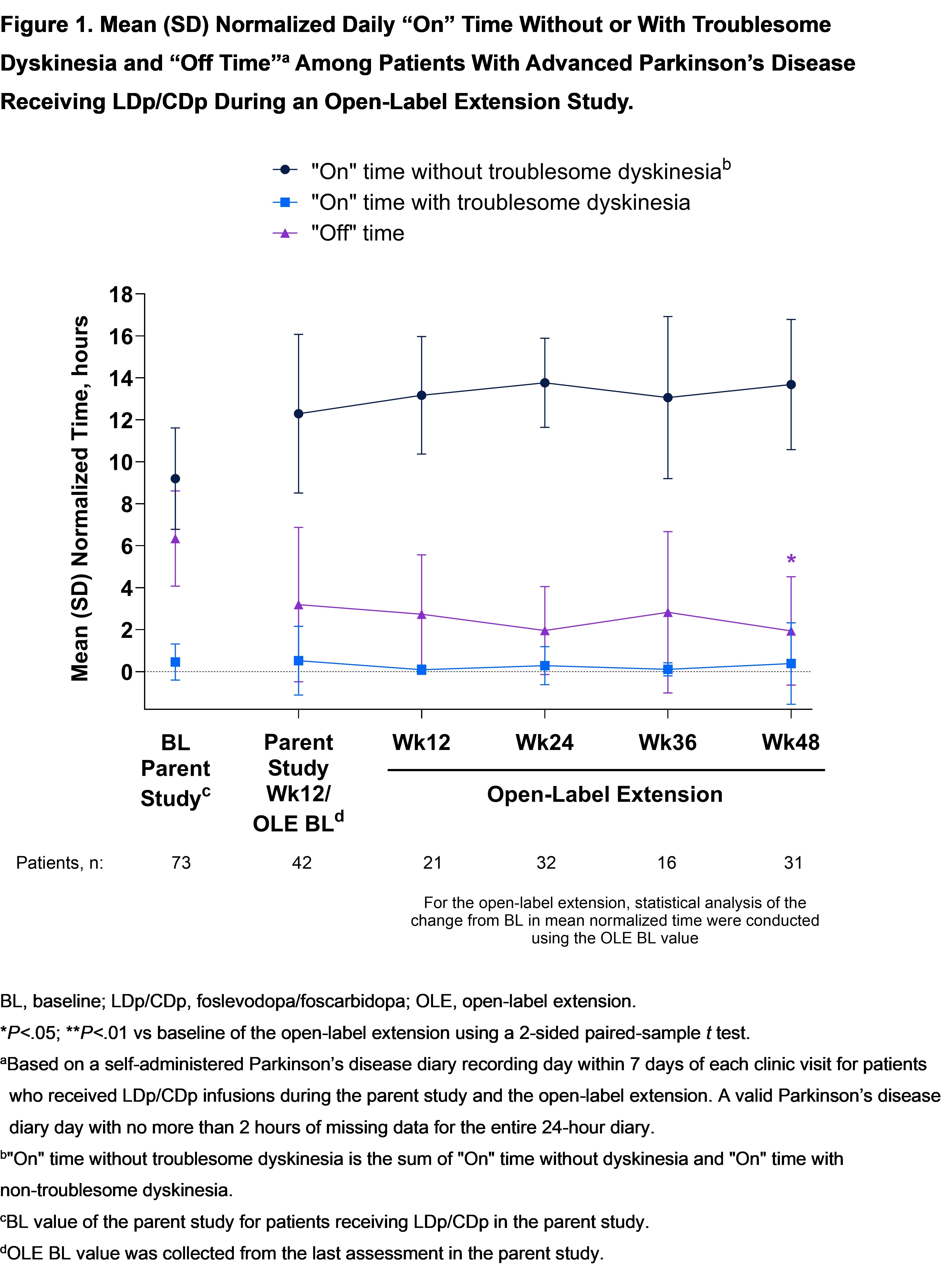
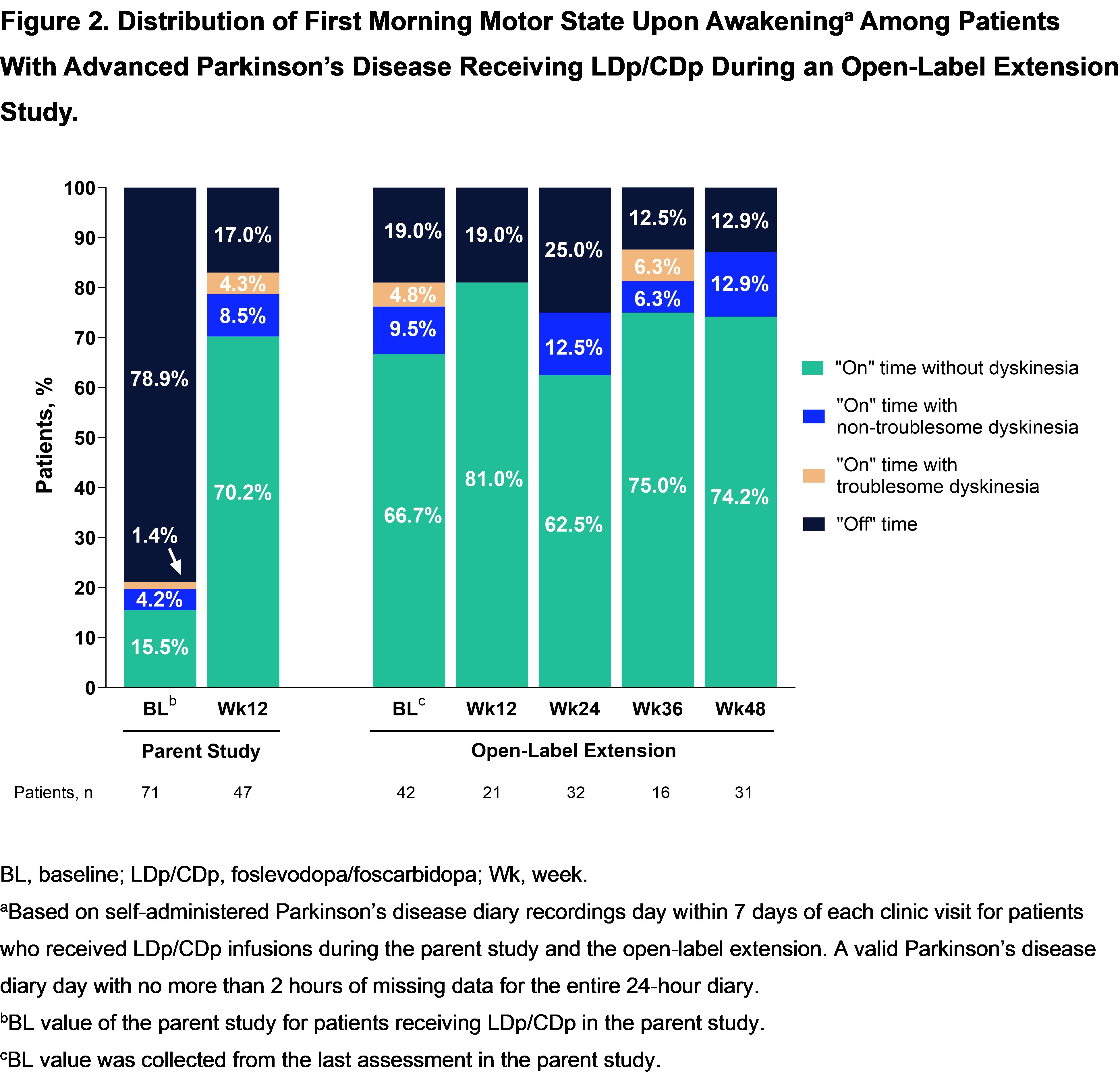
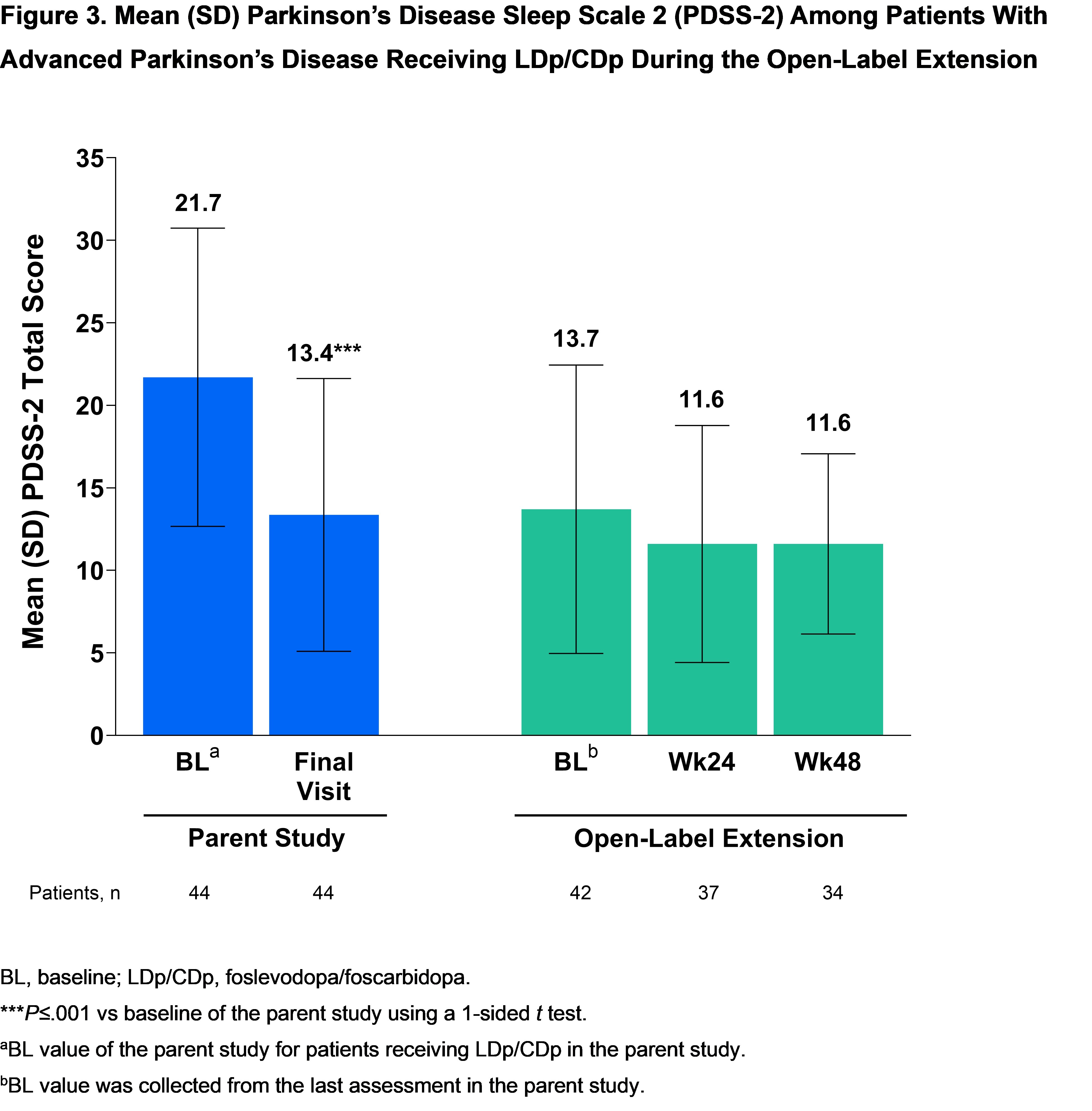
Results: 42 (88%) of 48 pts who received LDp/CDp and completed the parent study enrolled in the OLE; 35 (83%) are ongoing. Mean (SD) LDp/CDp exposure duration was 372.1 (111.6) days. The most common reason for study discontinuation was AE 3 [7%]). 38 (91%) pts experienced at least 1 AE; most were non-serious and mild or moderate. The most common (>10%) AEs were infusion site AEs [table]. Treatment-related improvements in “On” and “Off” time [figure1], morning akinesia (“Off” upon awakening) [figure2], and sleep [figure3] observed during the first 12-weeks of the parent study were sustained through week 48 of the OLE.
Conclusion: In combination with previously reported long-term data (NCT04379050) [2], these interim OLE results show that LDp/CDp continues to be generally well tolerated and treatment-related improvements in motor fluctuations, morning akinesia, and sleep are sustained over longer treatment durations.
Table
Figure 1
Figure 2
Figure 3
References: 1. Soileau MJ, Aldred J, Budur K, et al. Lancet Neurol. 2022;21:1099–1109.
2. Fung V, Aldred J, Bergquist F, et al. Mov Disord. 2023;38(suppl 1):Abstract 54. https://www.mdsabstracts.org/abstract/open-label-extension-study-of-long-term-safety-and-tolerability-of-foslevodopa-foscarbidopa-for-treatment-of-advanced-parkinsons-disease/. Accessed February 15, 2024.
To cite this abstract in AMA style:
R. Hauser, V. Fung, T. Kimber, K. Klos, M. Facheris, A. Jeong, J. Jia, A. Spiegel, M. Soileau. Long-Term Safety and Efficacy of Foscarbidopa/Foslevodopa in Patients with Advanced Parkinson’s Disease: Results From an Ongoing Phase 3 Open-Label Extension with Up to 1 Year Follow-Up [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/long-term-safety-and-efficacy-of-foscarbidopa-foslevodopa-in-patients-with-advanced-parkinsons-disease-results-from-an-ongoing-phase-3-open-label-extension-with-up-to-1-year-follow-up/. Accessed April 20, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-safety-and-efficacy-of-foscarbidopa-foslevodopa-in-patients-with-advanced-parkinsons-disease-results-from-an-ongoing-phase-3-open-label-extension-with-up-to-1-year-follow-up/