Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate long-term motor symptom control and improvement in quality of life (QoL) with foslevodopa/foscarbidopa (LDp/CDp) compared with baseline (BL) values from the parent study among people with advanced Parkinson’s disease (aPD) enrolled in an open-label extension (OLE).
Background: As PD progresses oral levodopa (LD)/carbidopa (CD) is unable to provide adequate symptom control contributing to disabling motor fluctuations and poor QoL. LDp/CDp is a soluble formulation of LD/CD prodrugs administered as a 24‑h/day continuous subcutaneous infusion. In a 52‑week open-label phase 3 study (NCT03781167), LDp/CDp improved motor fluctuations and QoL from the first post-BL visit through week 52 [1]. Patients who completed that study were eligible to enroll in a 96‑week OLE (NCT04379050).
Method: At BL of the parent study, patients were aged ≥30 years with LD-responsive, inadequately controlled (≥2.5 hours “Off” time/day) idiopathic aPD. LDp/CDp infusions are individually optimized at approximately 600–4250 mg LD equivalents/24 hour. Data are presented through week 72 of the OLE (interim cutoff, August 17, 2022). There was no multiplicity control; all P values are nominal.
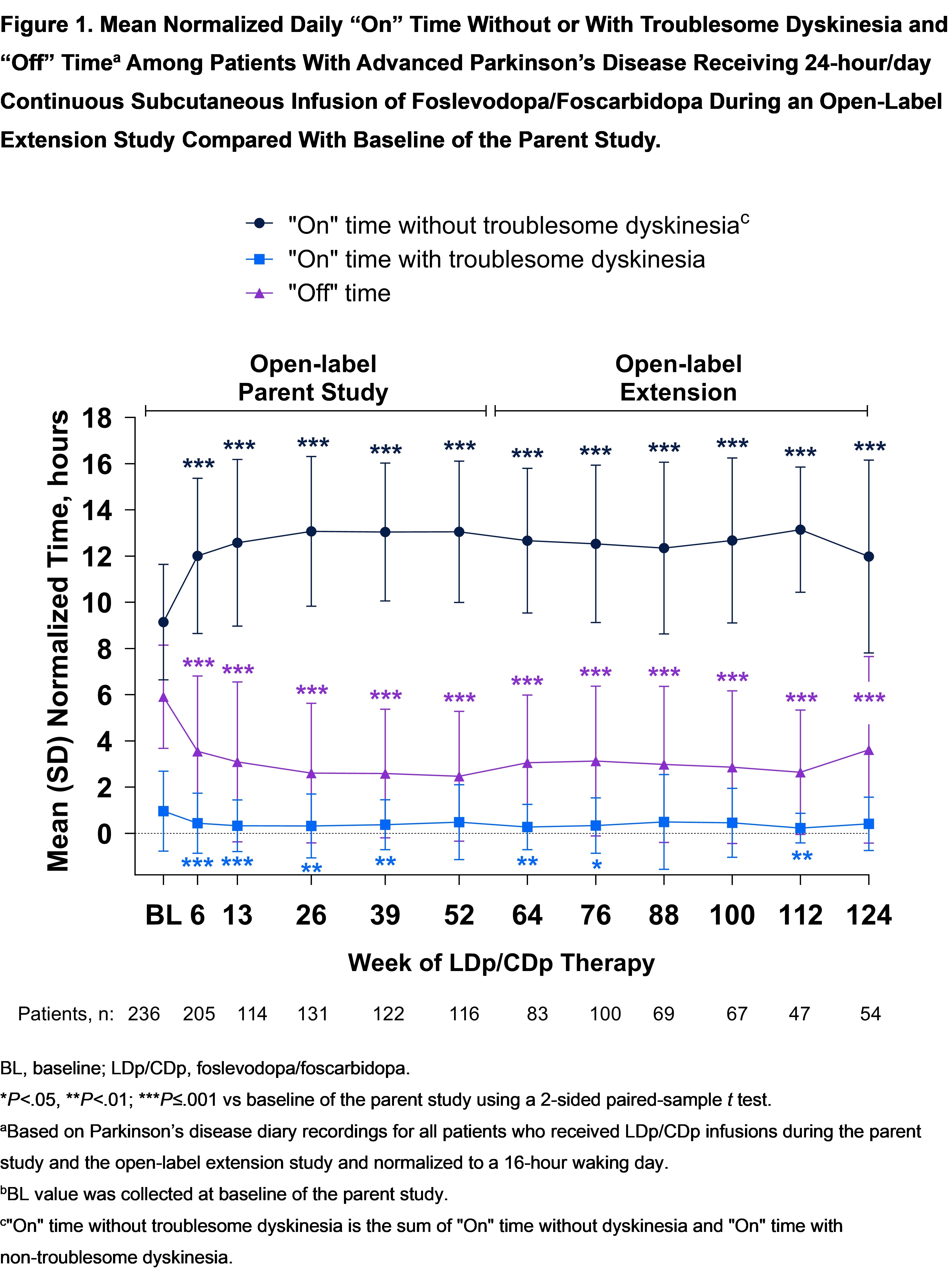
Results: 129 patients completed the parent study and enrolled in the OLE; 113 (88%) are ongoing. Improvements in “Off” and “On” time without troublesome dyskinesia were observed through 124 weeks of LDp/CDp therapy (52 weeks in the parent study plus 72 weeks of the OLE); mean (SD) change from BL of the parent study was −2.38 (4.52) hours for “Off” time (P≤.001) and 2.60 (4.51) hours for “On” time without troublesome dyskinesia (P≤.001) [figure1]. “On” time with troublesome dyskinesia remained low. The mean (SD) 39-item Parkinson’s Disease Questionnaire (PDQ-39) summary index score was improved compared with BL after 76 weeks of LDp/CDp therapy (29.0 [16.7] vs 33.2 [14.2] at BL of the parent study; P=.002) and improvement in QoL was sustained through 124 weeks. As previously described [2], most AEs in the parent and OLE studies were mild to moderate and non-serious, and the systemic LDp/CDp safety profile was consistent with that of oral LD/CD.
Conclusion: Combined with improvements observed through 52 weeks of the parent study, these results demonstrate sustained improvement in motor fluctuations and QoL through 124 weeks of LDp/CDp therapy.
Figure 1
References: 1. Aldred J, et al. Neuro Ther. 2023;12:1937–1958.
2. Fung V, et al. Mov Disord. 2023;38(suppl 1):Abstract 54. Available at: https://www.mdsabstracts.org/abstract/open-label-extension-study-of-long-term-safety-and-tolerability-of-foslevodopa-foscarbidopa-for-treatment-of-advanced-parkinsons-disease/. Accessed February 7, 2024
To cite this abstract in AMA style:
F. Bergquist, J. Aldred, C. Carroll, E. Danielsen, M. Facheris, R. Gupta, A. Jeong, A. Spiegel, V. Fung. Long-term Safety and Efficacy for Motor Fluctuations and Quality of Life With Foslevodopa/Foscarbidopa in Patients With Advanced Parkinson’s Disease: Interim Results From an Ongoing Open Label Extension [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/long-term-safety-and-efficacy-for-motor-fluctuations-and-quality-of-life-with-foslevodopa-foscarbidopa-in-patients-with-advanced-parkinsons-disease-interim-results-from-an-ongoing-open-label/. Accessed October 16, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-safety-and-efficacy-for-motor-fluctuations-and-quality-of-life-with-foslevodopa-foscarbidopa-in-patients-with-advanced-parkinsons-disease-interim-results-from-an-ongoing-open-label/