Category: Parkinson’s Disease: Clinical Trials
Objective: To report long-term (5-year and 11-year) neuropsychological outcomes from the deep brain stimulation (DBS) in early-stage Parkinson’s disease (PD) pilot clinical trial.
Background: The pilot trial randomized 30 early-stage PD patients (medication duration 0.5-4 years; without dyskinesia/motor fluctuations) to receive optimal drug therapy alone (early ODT) or subthalamic nucleus (STN) DBS plus ODT (early DBS+ODT; NCT00282152; IDEG050016).
Method: Two primary analyses were conducted: (1) 5-years of follow-up (IRB040797), n=28 (n=14 early ODT, n=14 early DBS+ODT); (2) 11-years of follow-up (IRB180766), n=12 (n=8 early ODT, n=4 early DBS+ODT). Linear mixed effects models for the 5-year and 11-year analyses compared the overall trend in outcomes for randomization groups (fixed effects: assigned treatment and year; random effect: subject) to account for repeated measures. All subjects who completed the 11-year study visit were also pooled to evaluate change from baseline to 11 years using Wilcoxon signed-rank tests.
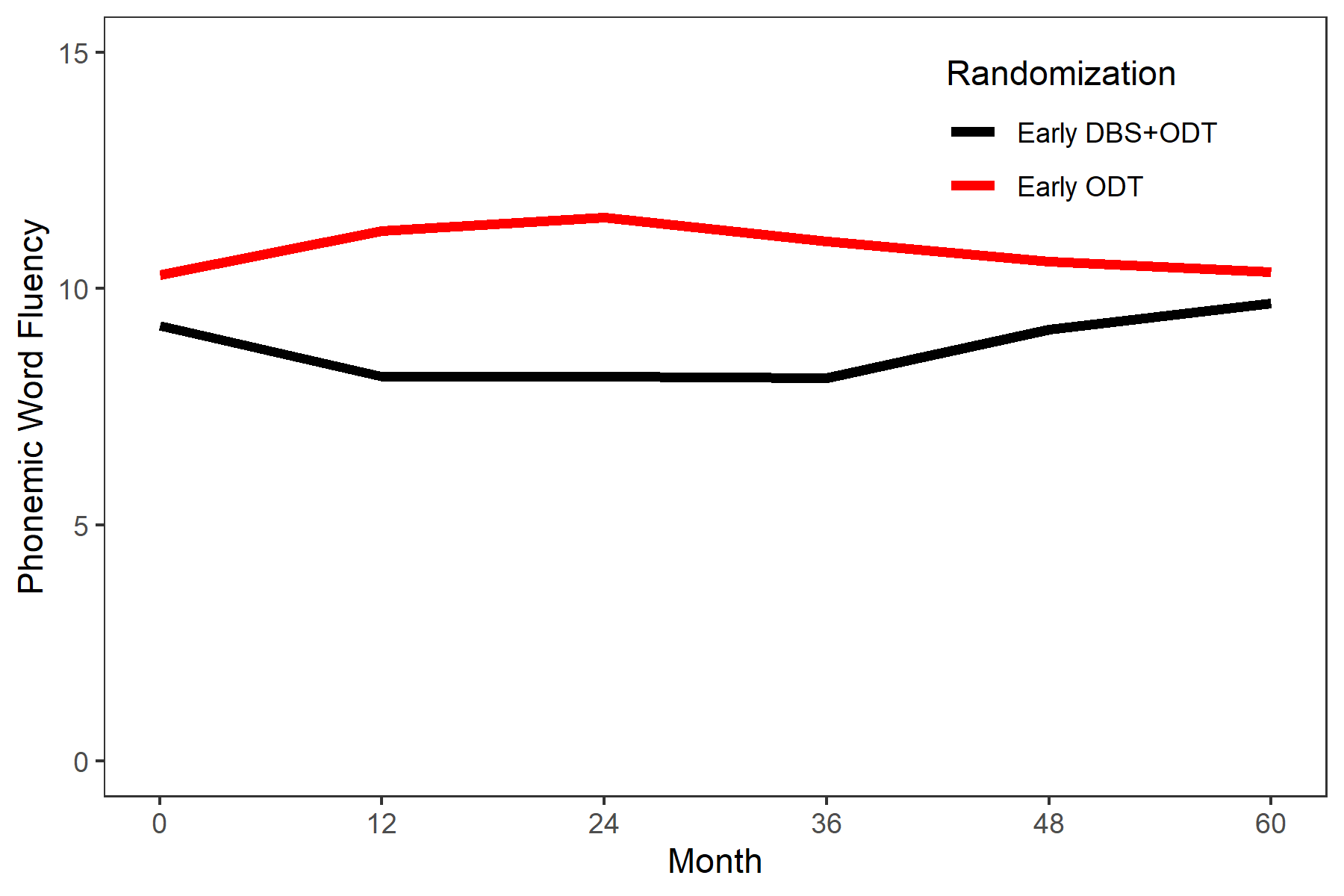
Results: Five-year analysis: verbal fluency (phonemic; Figure 1) & delayed word recall (WMS-III Word List II) favored early ODT subjects but did not reach statistical significance (P=0.08, P=0.07, respectively). These trends were no longer present in a sensitivity analysis that excluded two early DBS+ODT subjects with SAEs after surgery [1] (P=0.20 for both). Eleven-year analysis: there were no significant differences between randomization groups in the small cohort remaining at the 11-year follow-up visit. Across all PD patients who completed the 11-year visit (independent of randomization), there was significant decline in Stroop Color and Color-Word and Purdue Pegboard from baseline to 11 years (FDR-adjusted P=0.05).
Conclusion: This is the first study to report long-term cognitive outcomes of DBS in early-stage PD. Modest reductions in phonemic verbal fluency present through two years of follow-up for early DBS+ODT subjects largely resolved as PD progressed (Figure 1). No additional cognitive domains were worse for early DBS+ODT subjects compared to standard care subjects. More study is needed to understand the long-term neuropsychological outcomes of early DBS, and the FDA has approved a prospective, randomized, double-blind phase 3 clinical trial evaluating safety and efficacy of DBS in early-stage PD (IDEG050016).
References: [1] Tramontana MG, Molinari AL, Konrad PE, Davis TL, Wylie SA, Neimat JS, May AT, Phibbs FT, Hedera P, Gill CE, Salomon RM, Wang L, Song Y, Charles D (2015) Neuropsychological effects of deep brain stimulation in subjects with early stage Parkinson’s disease in a randomized clinical trial. J Parkinsons Dis 5, 151–163.
To cite this abstract in AMA style:
M. Hacker, M. Tramontana, J. Meystedt, M. Turchan, K. Harper, B. Eoff, R. Fan, F. Ye, T. Davis, P. Konrad, D. Charles. Long-Term Neuropsychological Outcomes of Deep Brain Stimulation in Early-Stage Parkinson’s Disease [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/long-term-neuropsychological-outcomes-of-deep-brain-stimulation-in-early-stage-parkinsons-disease/. Accessed December 19, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-neuropsychological-outcomes-of-deep-brain-stimulation-in-early-stage-parkinsons-disease/