Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: The objective of this study was to summarize evidence evaluating long-term (i.e. ≥ 12 months)efficacy of levodopa carbidopa intestinal gel (LCIG) on “off” time, through a systematic literature review.
Background: A large number of studies have demonstrated the effect of LCIG in significantly reducing “off-time” in patients with advanced Parkinson’s disease (APD). However the length of follow-up in the studies is variable and may range from 3 months to over 5 years.
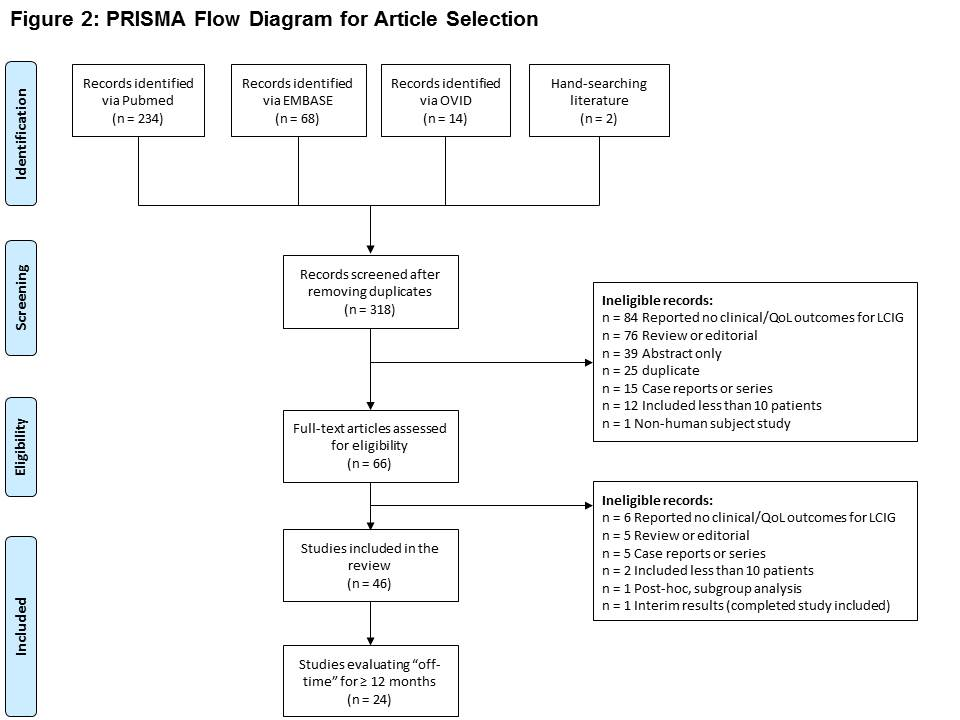
Method: As part of a wider review, studies evaluating the safety and efficacy of LCIG were identified from January 1, 2000 through September 1, 2018 from PubMed, EMBASE, and Ovid using a comprehensive search strategy [Figure 1] and a priori selection criteria [Table 1]. Search was restricted to peer-reviewed articles published in English. From the identified studies, only studies with a long term follow-up (i.e. ≥ 12 months) were included in this review. Screening and data extraction was done separately by 2 independent reviewers and results were matched. Qualitative synthesis of the data was conducted.
Results: This review included 24 studies which evaluated long term efficacy of LCIG on “off” time [Figure 2]. Out of 24 studies, 50% were multicenter and ~42% had a sample size of ≥ 50 patients. Mean disease duration at baseline ranged from 10 to 23 years (n=22). Efficacy of LCIG based on “off”-time reduction was evaluated using UPDRS-IV item 39 (n=15), PD diary (n=6), physician assessment (n=3), and MDS-UPDRS item 4.3 (n=2). Mean baseline off-time ranged from 2.1 – 8 hours/waking day. Mean % reduction in “off” time at end of follow-up ranged from 37% to 84% (compared to baseline). Stratified analysis of “off time” demonstrated mean relative reduction of 18%-78% at 3-6 months and upto 83% reduction at 3-5 years of follow-up. Attrition rate in the studies varied by the length of follow-up. Majority of adverse events in patients receiving LCIG involved device and procedure-related issues and were observed during the first 3 months.
Conclusion: Studies evaluating long term efficacy of LCIG on “off” time demonstrated early and sustained response to LCIG through end of follow-up. Analysis assessing the extent of heterogeneity between studies (including patient characteristics, attrition rate, study settings, and length of follow-up) is warranted in the future.
To cite this abstract in AMA style:
A. Antonini, P. Odin, R. Pahwa, J. Aldred, A. Alobaidi, Y. Jalundhwala, P. Kukreja, L. Bergmann, S. Inguva, Y. Bao, K. Chaudhuri. Long-term impact of Levodopa Carbidopa Intestinal Gel (LCIG) on reducing “Off” time: Results from a systematic review of studies with ≥ 12 months of follow-up [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/long-term-impact-of-levodopa-carbidopa-intestinal-gel-lcig-on-reducing-off-time-results-from-a-systematic-review-of-studies-with-%e2%89%a5-12-months-of-follow-up/. Accessed December 26, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-impact-of-levodopa-carbidopa-intestinal-gel-lcig-on-reducing-off-time-results-from-a-systematic-review-of-studies-with-%e2%89%a5-12-months-of-follow-up/