Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate the long-term evolution of clinical and economic outcomes and treatment patterns of patients with advanced Parkinson’s disease (PD) who have symptoms that are not adequately controlled by their current therapy.
Background: In patients with advanced PD, motor symptoms become increasingly difficult to control with oral medications. Few studies have evaluated the disease progression of patients with motor fluctuations inadequately controlled by oral therapies in an observational manner.
Method: The 24-month PROSPECT (Prospective Observational Study to evaluate the disease Progression and burdEn of disease of PD patients inadequately Controlled by conventional Therapy) study is an ongoing international investigation of adult patients (aged ≥30 years) with idiopathic PD. Eligible patients had inadequately controlled motor symptoms (≥2.5-hours/day “Off” time) despite trial and optimization of available oral/transdermal/sublingual/inhalable medication. Patients could not be receiving device-aided therapy (DAT) at enrollment. This interim analysis was performed after all patients reached month 12.
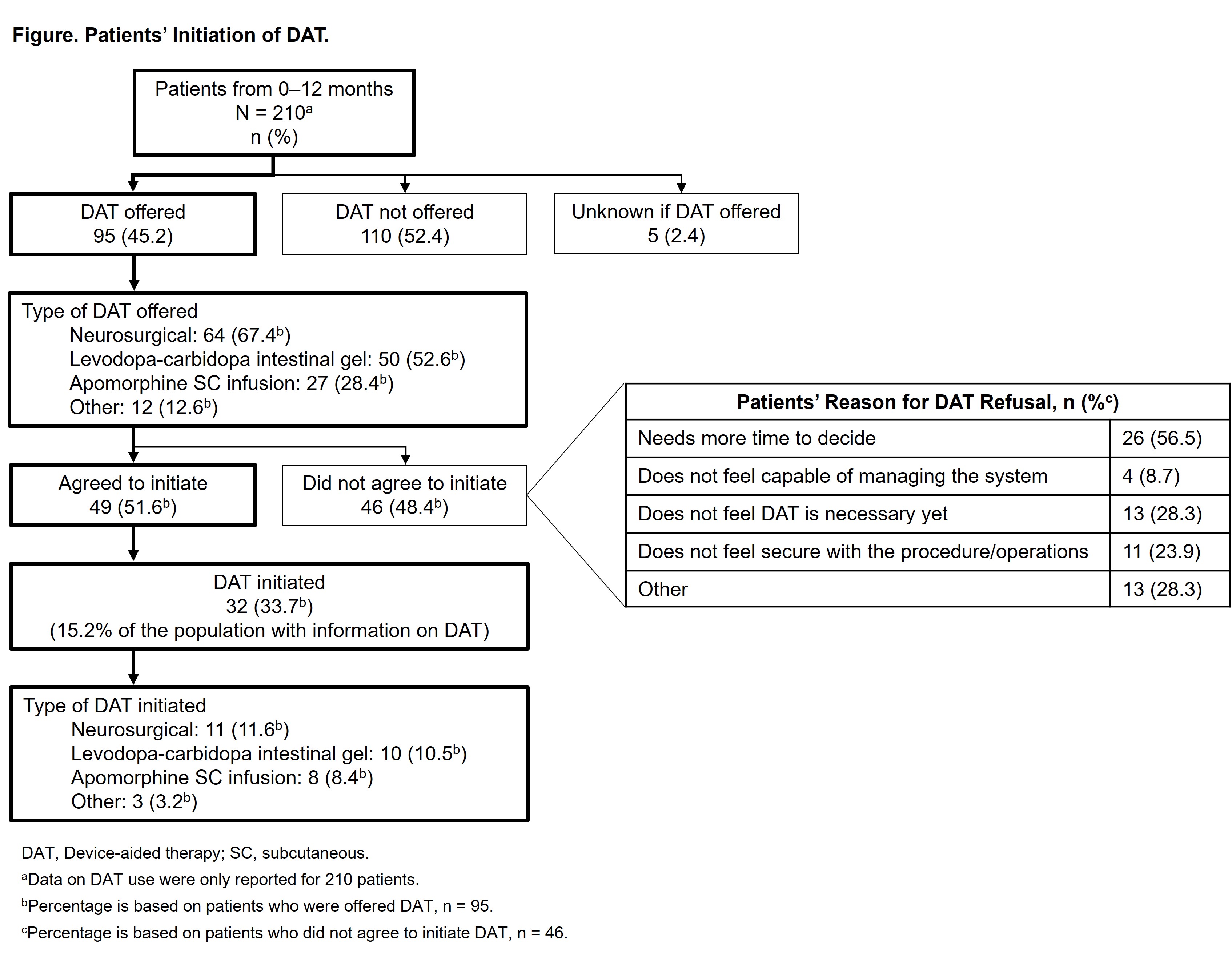
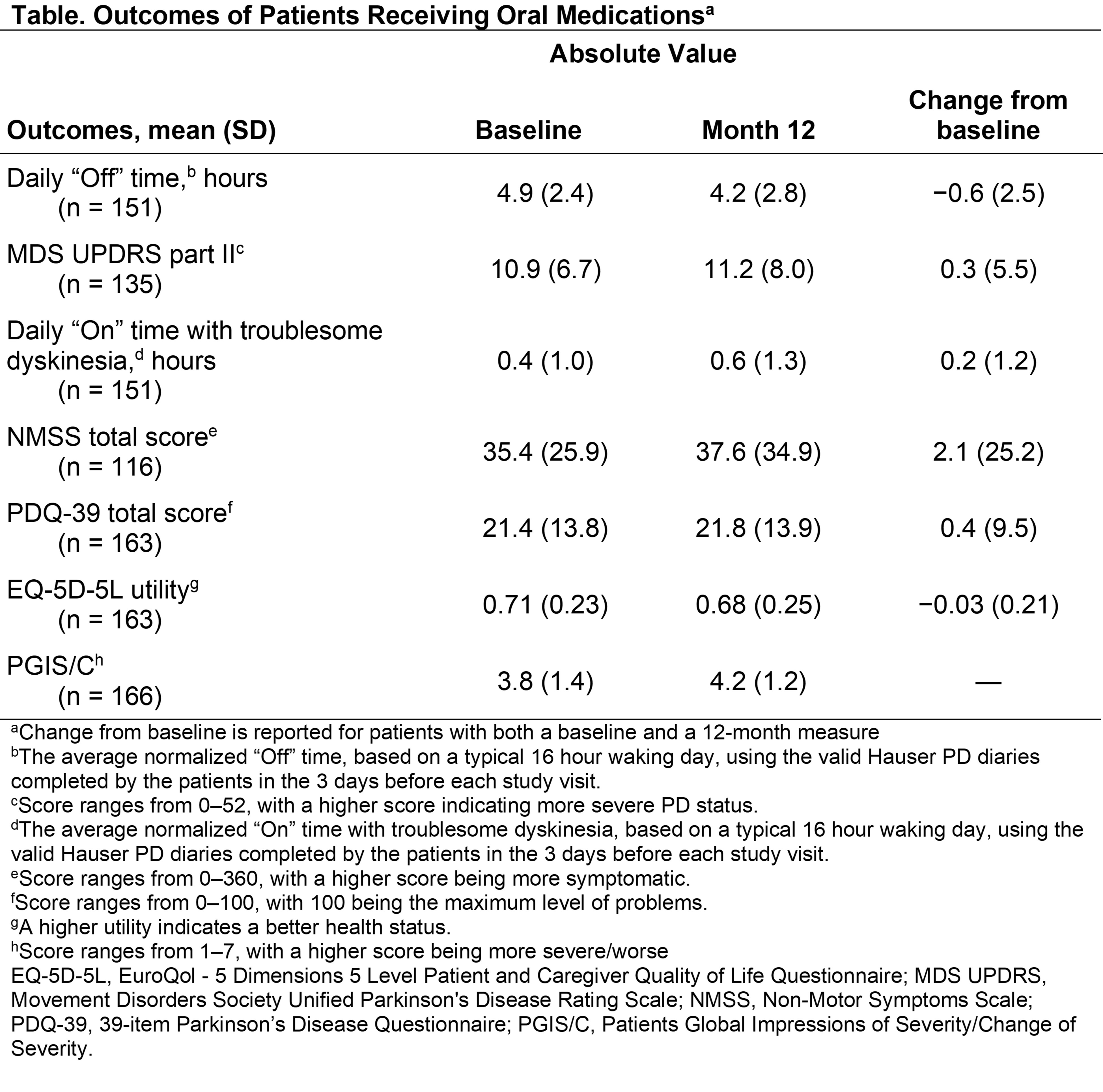
Results: This second interim analysis includes 227 patients with a mean (SD) age of 67.9 (9.5) years, mean (SD) PD duration of 8.9 (5.4) years, and mean (SD) daily “Off” time of 5.0 (2.5) hours. At month 12, patients on only oral medication (n=195) had little change in “Off” time (mean [SD] change from baseline to month 12: −0.6 [2.5] hours/day), reported no change or slight worsening on the Patients Global Impression of Change of Severity score (mean [SD], 4.2 [1.2]), and had worsening nonmotor symptoms [Table]. DAT was offered to fewer than half (45.2%) of patients (n/N=95/210) by month 12 [Figure]. Of the 95 patients offered DAT, 32 (33.7%) initiated DAT (overall n/N=32/210, 15.2%). Some reasons for refusal of DAT cited by patients included more time needed to decide and the perception that DAT was unnecessary.
Conclusion: While patients had a considerable disease burden at the beginning of the study, the motor symptoms of patients treated with oral medications were still inadequately controlled (≥2.5-hours/day “Off” time) at month 12, with generally little change or worsening in nonmotor symptoms. Despite inadequate symptom control, over 80% of patients did not initiate DAT by month 12.
To cite this abstract in AMA style:
P. Sanchez Alonso, O. de Fabregues, A. Lehn, T. Oeda, F. Ory-Magne, D. Safarpour, L. Bergmann, P. Kukreja, K. Onuk, V. Fung. Long-term evolution of advanced Parkinson’s disease burden: second interim results from the international PROSPECT observational study [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/long-term-evolution-of-advanced-parkinsons-disease-burden-second-interim-results-from-the-international-prospect-observational-study/. Accessed December 20, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-evolution-of-advanced-parkinsons-disease-burden-second-interim-results-from-the-international-prospect-observational-study/