Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate the long-term effect of ADS-5102 on OFF time among Parkinson’s disease (PD) patients with levodopa-induced dyskinesia (LID).
Background: ADS-5102, the only drug FDA-approved for LID in PD, also significantly reduced OFF time in phase 3 trials, with improvements sustained over a 2-year, open-label trial (EASE LID 2, NCT02202551). Both OFF and dyskinesia cause disability and affect quality of life, so treating dyskinesia should not come at the expense of increased OFF, or vice versa. This analysis further characterizes the long-term effects of ADS-5102 on OFF by evaluating the distribution of patient MDS-UPDRS Part IV scores.
Method: EASE LID 2 enrolled patients with MDS-UPDRS Part IV (motor complications) scores for item 4.3 (time spent OFF) and item 4.4 (functional impact of OFF) obtained prior to first ADS-5102 initiation in either double-blind or open-label were included. Distribution of MDS-UPDRS Part IV scores were evaluated over 100 weeks of open-label treatment.
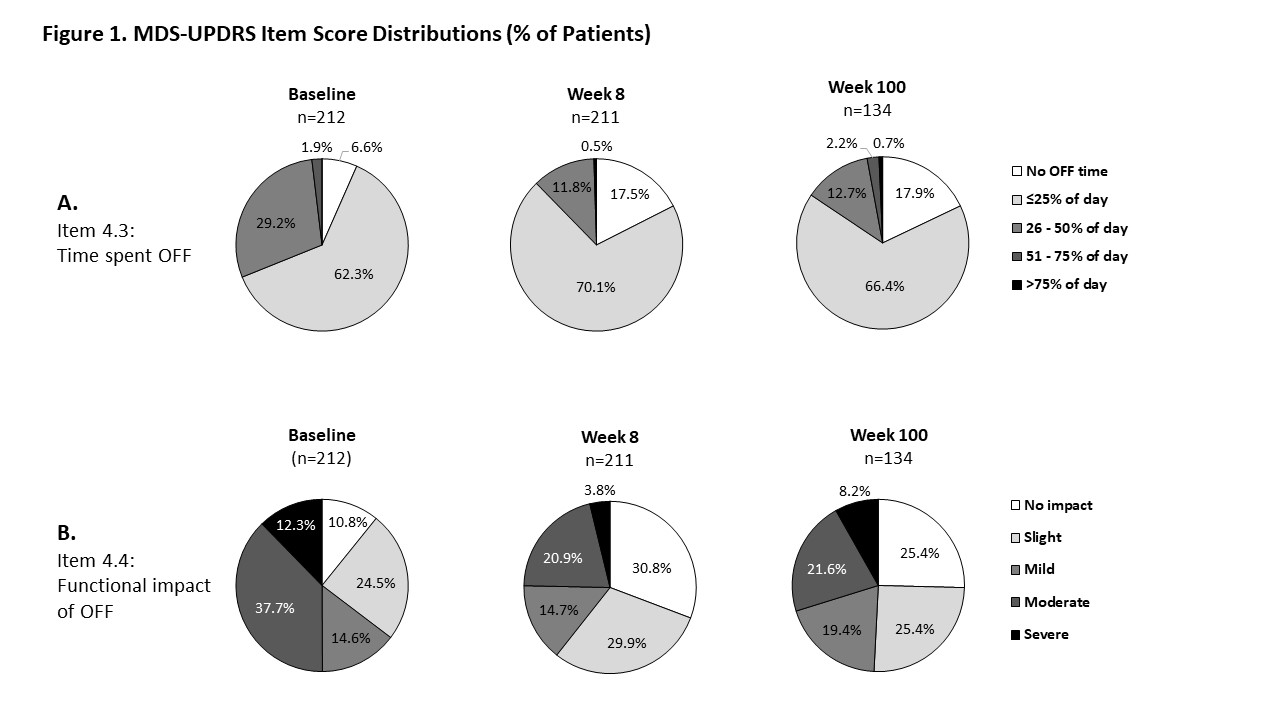
Results: Of 223 participants, 212 had an MDS-UPDRS Part IV score prior to ADS-5102 treatment in either double-blind or open-label. Of these, 6.2% reported no OFF time at baseline, 62.3% reported OFF time less than a quarter of the waking day, and 31.1% reported OFF time more than a quarter of the waking day. Overall, OFF time shifted toward improvement by Week 8 (first open-label visit), with the percentage of patients reporting no OFF time increasing to 17.5% at Week 8, and 17.9% at Week 100 [Figure 1]. Similarly, 10.8% of patients reported no impact of OFF on social interactions and daily activities at baseline, increasing to 30.8% at Week 8 and 25.5% at Week 100, and fewer patients reported mild or greater functional impact of OFF, decreasing from 64.6% pre-treatment to 39.4% at Week 8 and 49.2% at Week 100. MDS-UPDRS items 4.1 and 4.2, measuring daily time and functional impact of dyskinesia, also improved by Week 8 and remained favorable through Week 100.
Conclusion: In addition to the demonstrated improvement in dyskinesia, the percentage of patients reporting no OFF time and no impact of OFF on daily function more than doubled by the first on-treatment visit in this open-label trial and remained favorable at the 2-year trial endpoint. These results suggest a durable treatment effect of ADS-5102 on OFF time as well as dyskinesia.
To cite this abstract in AMA style:
C. Tanner, D. Chernick, A. Formella. Long-term effects of ADS-5102 (amantadine) extended release capsules on OFF symptoms in Parkinson’s disease patients with levodopa-induced dyskinesia: Analysis of EASE LID 2 trial [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/long-term-effects-of-ads-5102-amantadine-extended-release-capsules-on-off-symptoms-in-parkinsons-disease-patients-with-levodopa-induced-dyskinesia-analysis-of-ease-lid-2-trial/. Accessed October 21, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-effects-of-ads-5102-amantadine-extended-release-capsules-on-off-symptoms-in-parkinsons-disease-patients-with-levodopa-induced-dyskinesia-analysis-of-ease-lid-2-trial/