Category: Parkinson’s Disease: Clinical Trials
Objective: To assess the effects of lithium aspartate and lithium carbonate therapy for 24 weeks on serum NfL in Parkinson’s disease (PD).
Background: Lithium has a wide range of neuroprotective actions, has been effective in several PD animal models and may account for the decreased risk of PD in smokers.[1] Two promising PD disease-progression biomarkers are substantia nigra (SN) free water (FW) assessed by diffusion MRI and serum NfL.[2,3] Our group recently reported 24 weeks of lithium therapy to be associated with reductions (i.e. improvements) in SN FW in a small, pilot clinical trial.[4] Such findings suggest that lithium therapy may have disease-modifying effects in PD. Using frozen serum samples from the same pilot trial, we now assessed lithium’s effects on serum NfL.
Method: This open-label, pilot clinical trial randomized 16 PD patients to “high-dose” (n=5, lithium carbonate titrated to a serum level of 0.4-0.5mmol/L), “medium-dose” (n=6, 45mg/day lithium aspartate) or “low-dose” (n=5,15mg/day lithium aspartate) lithium therapy for 24-weeks. Three additional PD patients did not receive lithium and served as controls. Fasting blood samples were obtained at baseline, 12 and 24 weeks. Serum samples stored at -20 F were assessed for NfL in duplicate by Quanterix (Lexington, MA) using the SIMOA platform.
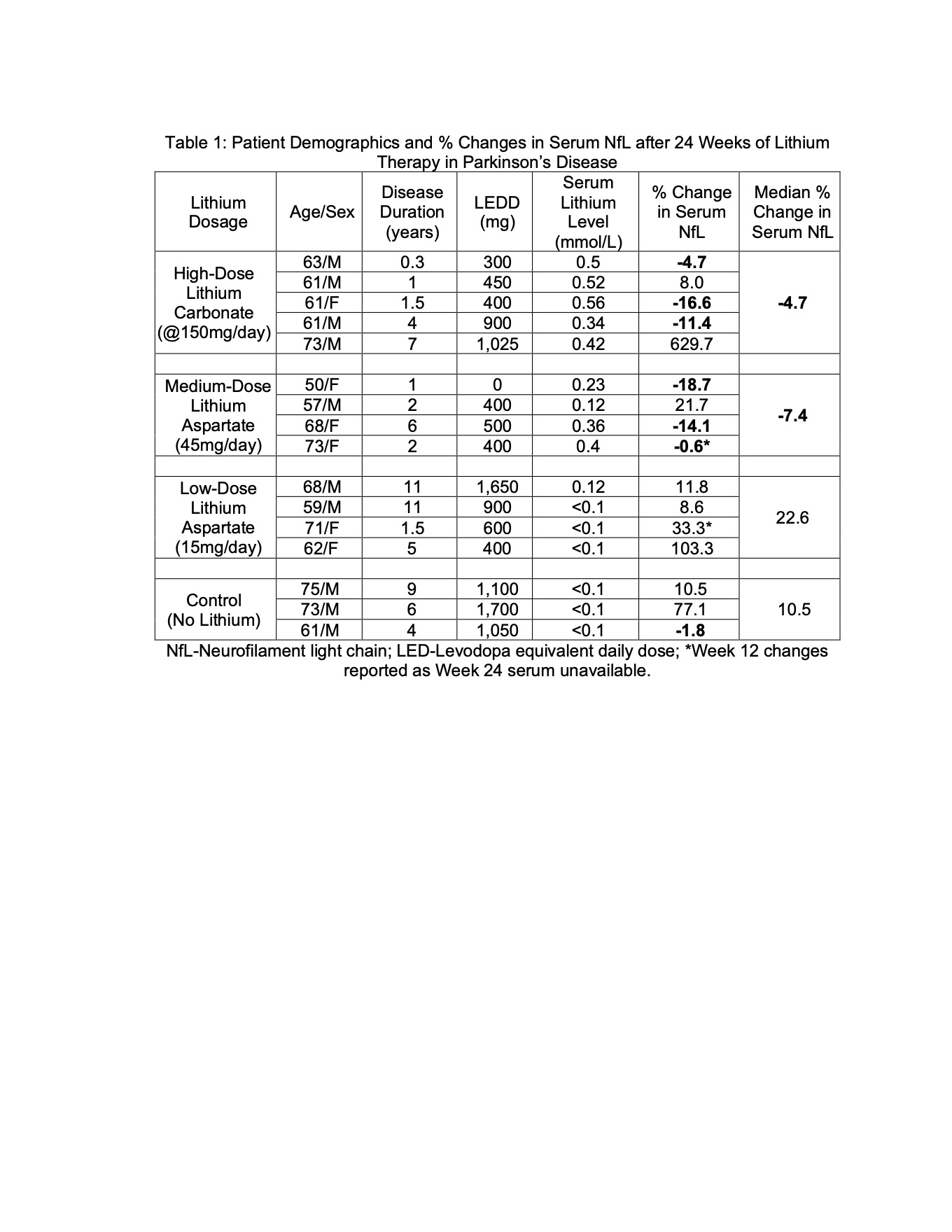
Results: Two of the six patients receiving medium-dose lithium aspartate therapy withdrew due to side effects. One of the low-dose lithium aspartate patients did not have baseline serum available. Baseline patient demographics and percent changes in serum NfL are summarized in Table 1. “High-dose” lithium carbonate and “medium-dose” lithium aspartate therapy were associated with median decreases in serum NfL of 4.7% and 7.4%, respectively. “Low-dose” lithium aspartate therapy and control patients showed median increases in serum NfL of 22.6% and 10.5%, respectively.
Conclusion: Lithium aspartate 45mg/day and lithium carbonate @150mg/day for 24 weeks were associated with median reductions in serum NfL. Larger controlled trials are necessary to determine if lithium significantly reduces serum NfL in PD, which would support a disease-modifying effect.
Table 1:
References: [1] Guttuso T, Jr., Russak E, De Blanco MT, Ramanathan M. Could high lithium levels in tobacco contribute to reduced risk of Parkinson’s disease in smokers? J Neurol Sci 2019;397:179-80. doi: 10.1016/j.jns.2019.01.009
[2] Burciu RG, Ofori E, Archer DB, et al. Progression marker of Parkinson’s disease: a 4-year multi-site imaging study. Brain 2017;140:2183-92. doi: 10.1093/brain/awx146
[3] Mollenhauer B, Dakna M, Kruse N, et al. Validation of Serum Neurofilament Light Chain as a Biomarker of Parkinson’s Disease Progression. Mov Disord 2020. doi: 10.1002/mds.28206
[4] Guttuso T, Jr., Shepherd R, Frick L, et al. Lithium’s effects on therapeutic targets and MRI biomarkers in Parkinson’s disease: A pilot clinical trial. IBRO Neurosci Rep 2023;14:429-34. doi: 10.1016/j.ibneur.2023.05.001
To cite this abstract in AMA style:
T. Guttuso, JR., R. Shepherd. Lithium Therapy Associated with Reductions in Serum Neurofilament Light Chain (NfL) in Parkinson’s Disease. [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/lithium-therapy-associated-with-reductions-in-serum-neurofilament-light-chain-nfl-in-parkinsons-disease/. Accessed December 6, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/lithium-therapy-associated-with-reductions-in-serum-neurofilament-light-chain-nfl-in-parkinsons-disease/