Session Information
Date: Monday, October 8, 2018
Session Title: Parkinson's Disease: Pathophysiology
Session Time: 1:15pm-2:45pm
Location: Hall 3FG
Objective: To investigate the relationship between slow wave activity (SWA) of non-rapid eye movement (NREM) sleep, which underlies adjustment of cortical excitability, and levodopa-induced dyskinesias (LIDs)
Background: The spectrum of clinical symptoms in Parkinson’s disease is susceptible to changes through the course of the disease. Levodopa therapy successfully controls motor symptoms for several years and then induces motor fluctuation and abnormal involuntary movements, i.e., levodopa-induced dyskinesias (LIDs) that have been associated with abnormal cortical plasticity
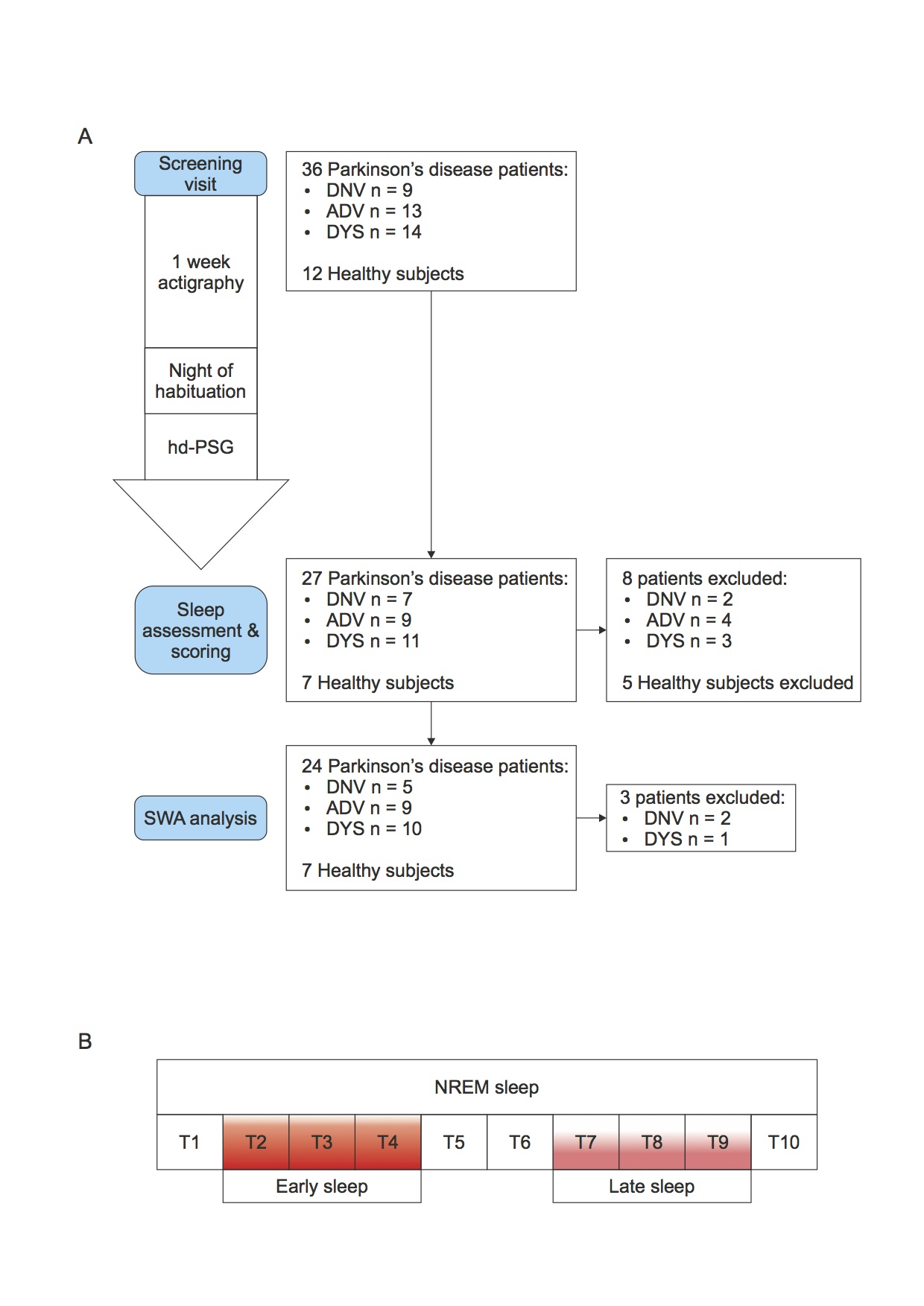
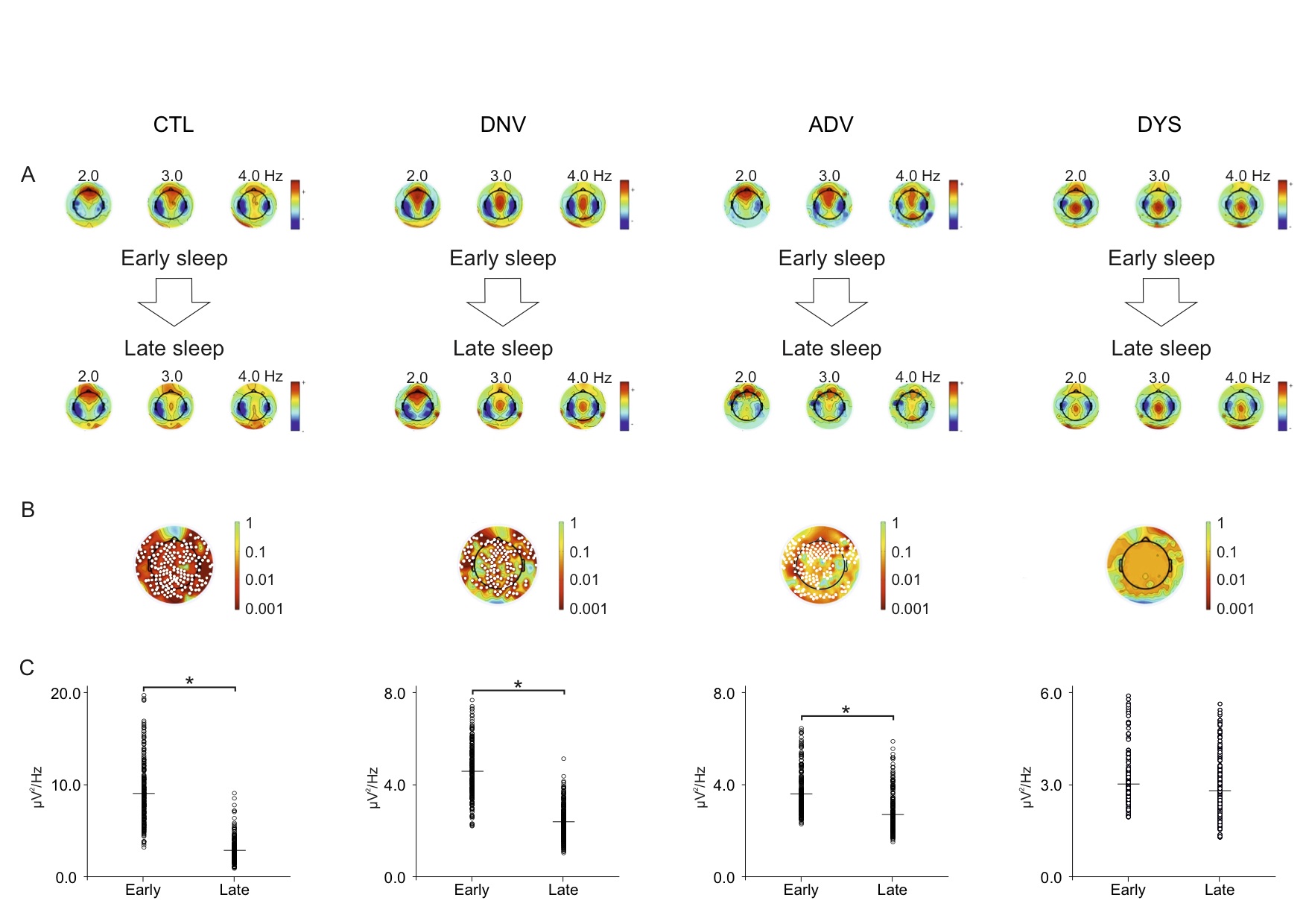
Methods: Thirty-six patients with different stages of Parkinson’s disease underwent whole-night video polysomnography high density EEG (vPSG-hdEEG), preceded by 1-week actigraphy. To represent the broad spectrum of the disease, patients were divided into three groups by disease stage-(i) de novo (DNV; n = 9), (ii) advanced (ADV; n = 13), and (iii) dyskinetic (DYS; n = 14)-and were compared to an age-matched control group (CTL; n = 12). The SWA-NREM content of the PSG-hdEEG was subdivided into 10 equal segments. The 2nd, 3rd, and 4th segments and the 7th, 8th, and 9th segments were designated as early and late sleep
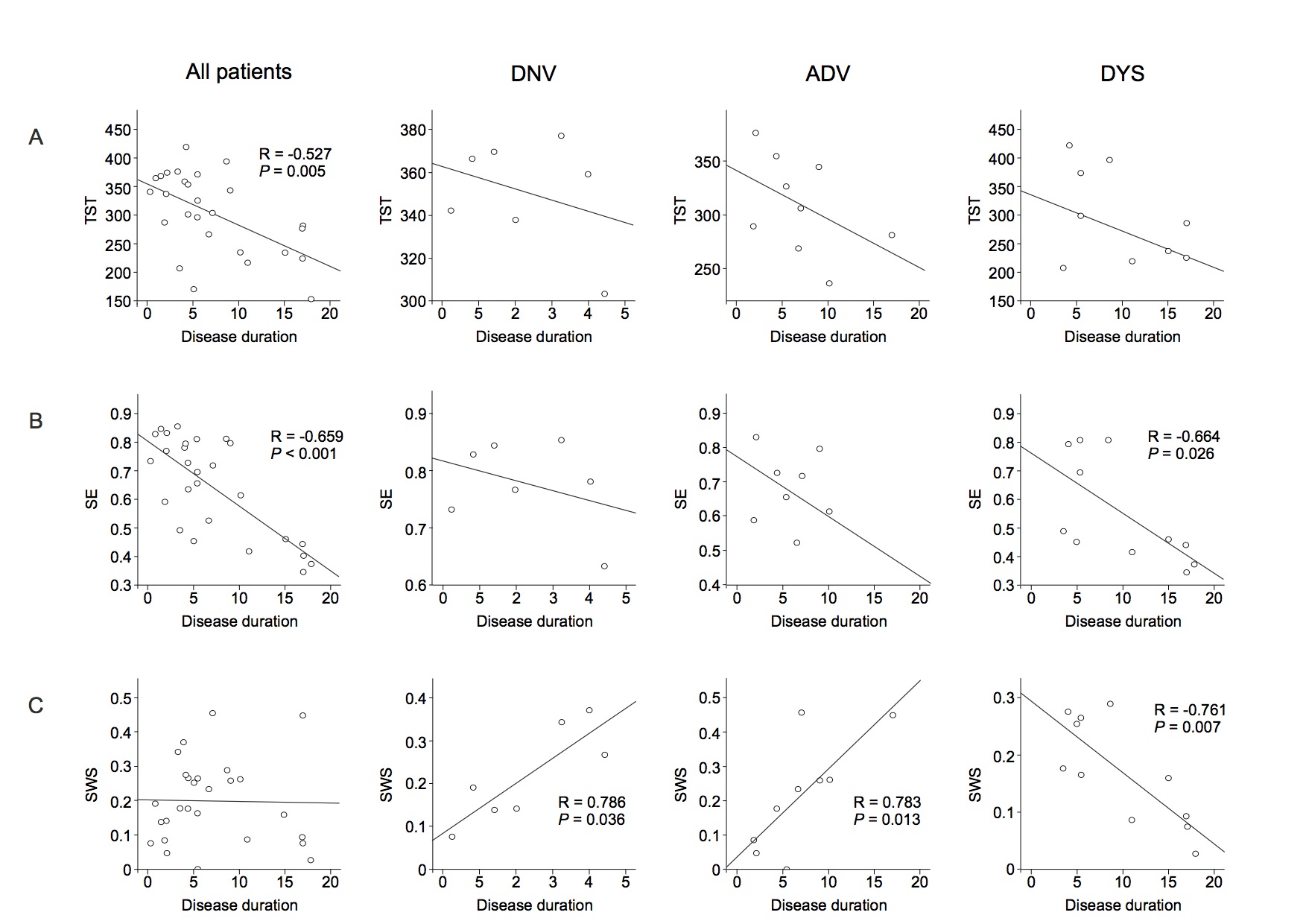
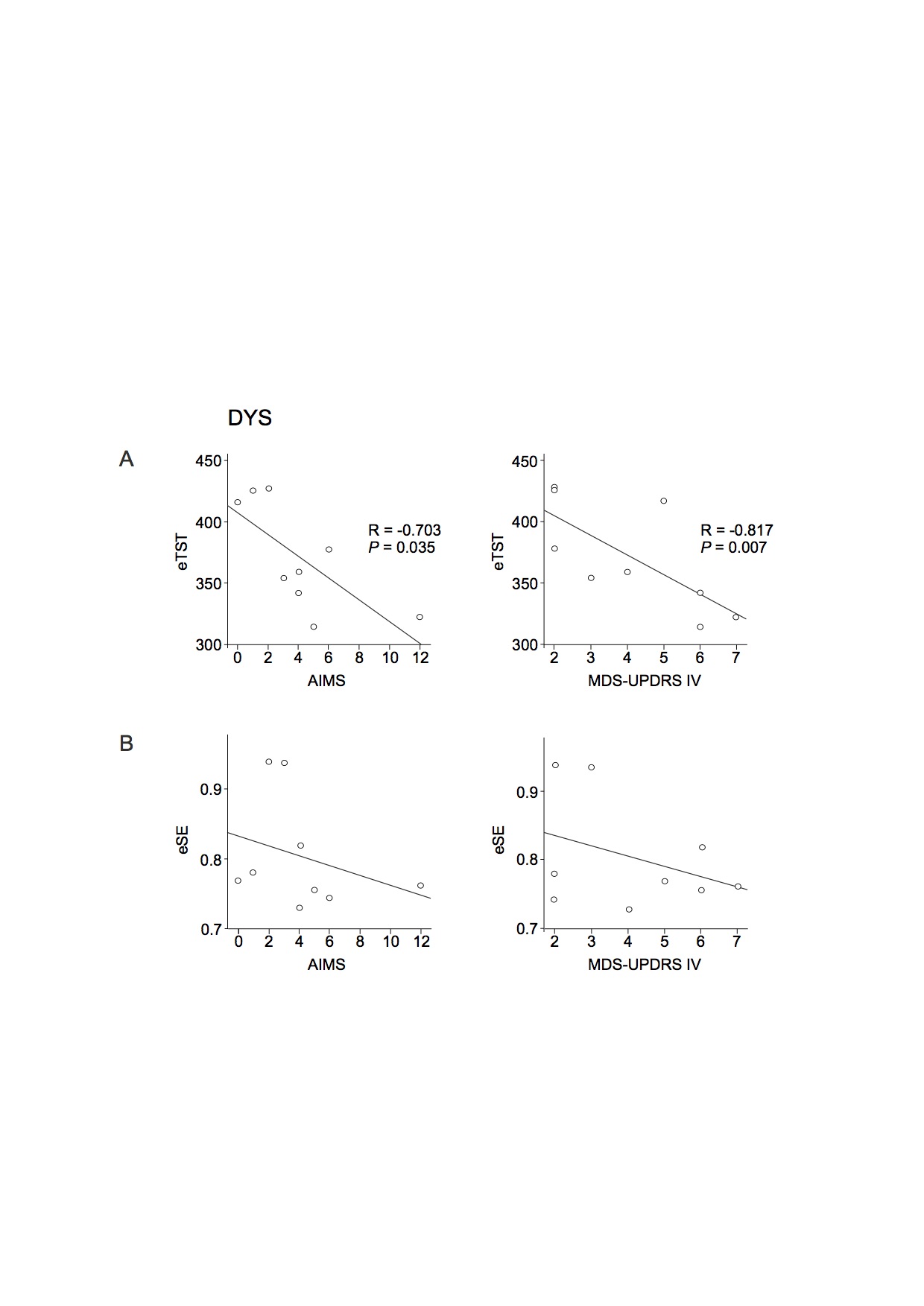
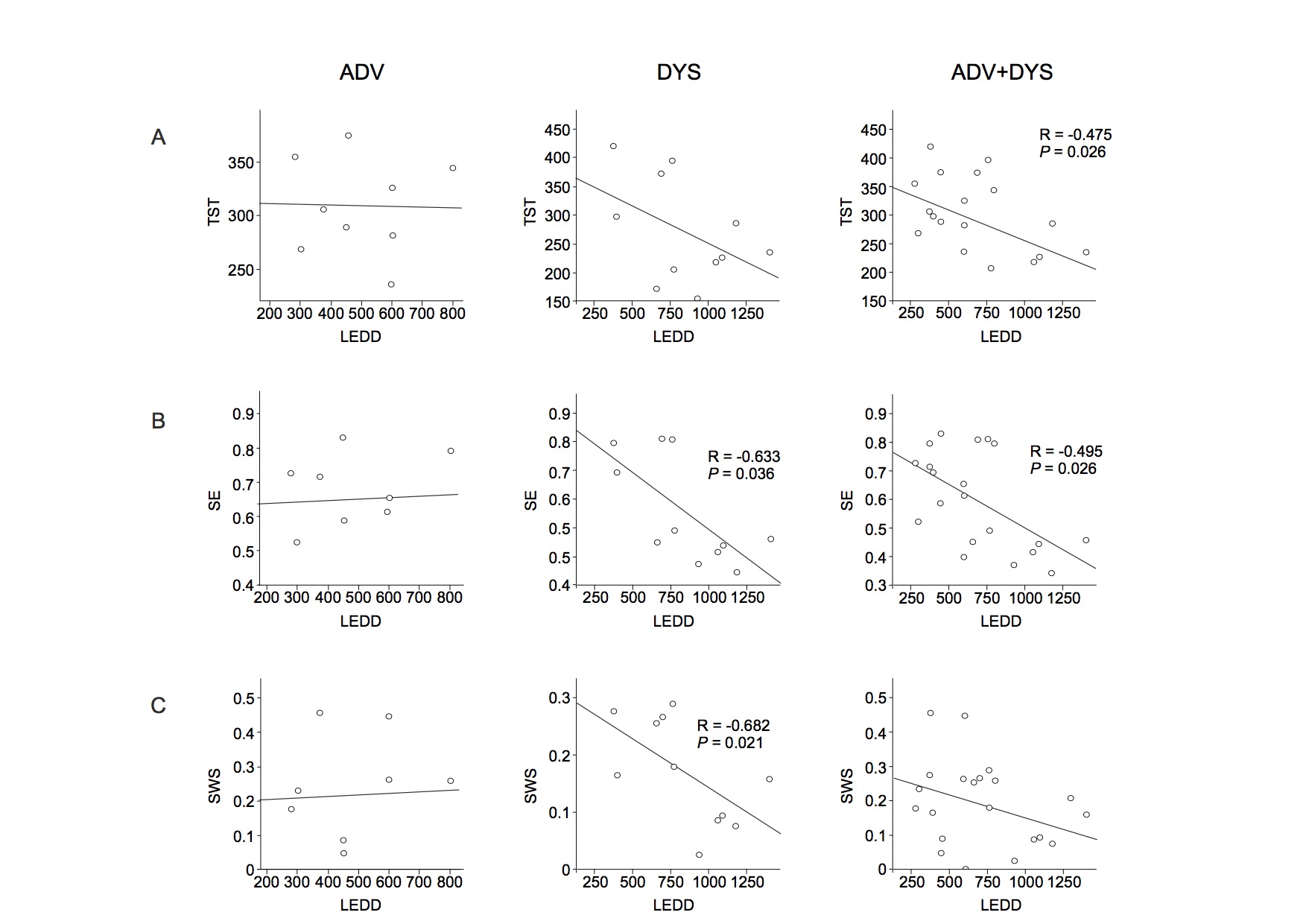
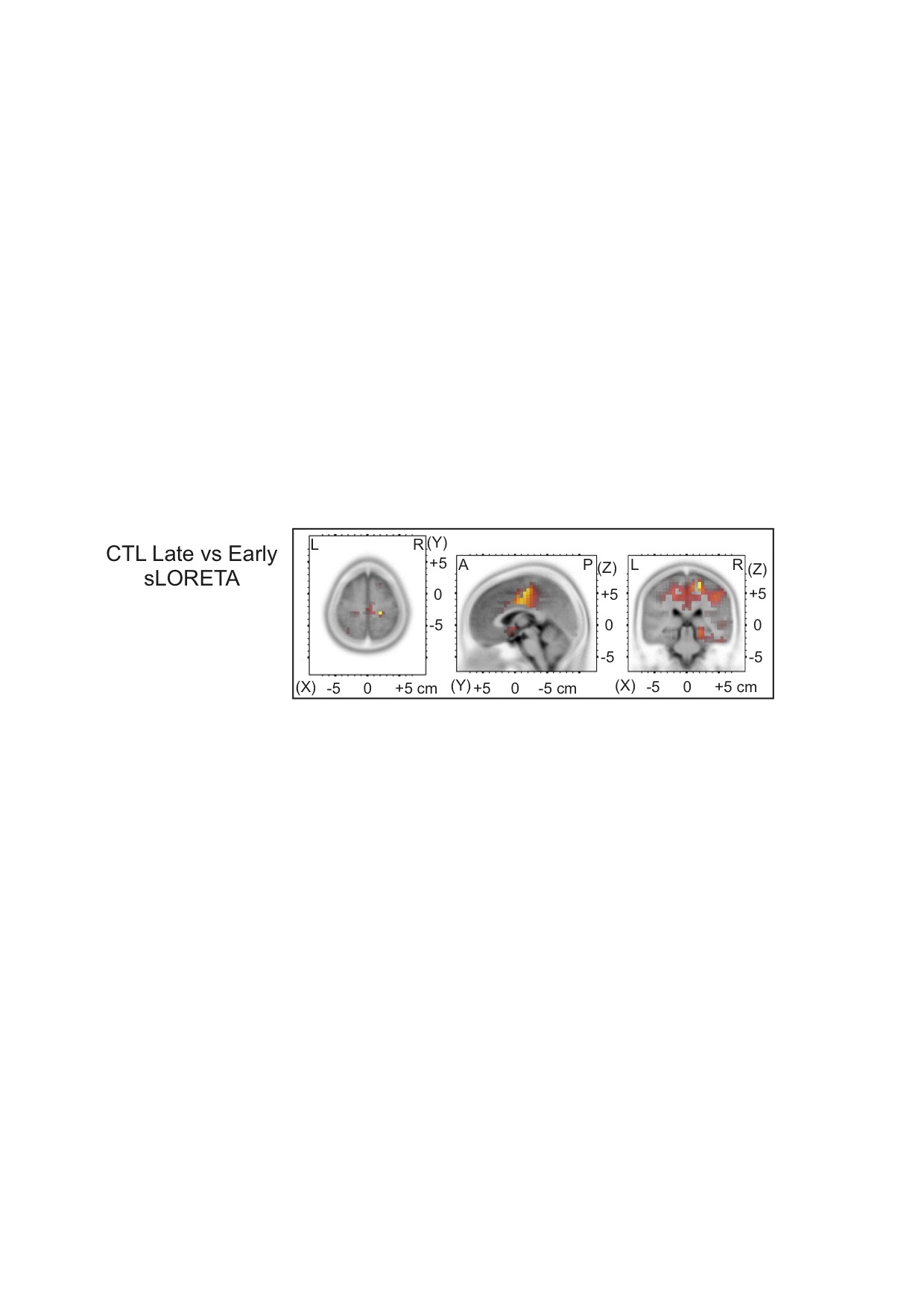
Results: There was a negative correlation between both total sleep time (TST) and sleep efficiency (SE) and disease duration in all patients. Of note, slow wave sleep (SWS) and disease duration were positively correlated in the DNV and ADV groups and negatively correlated in the DYS group. TST and SE were negatively correlated with the levodopa-equivalent daily dose (LEDD) in all patients with motor fluctuations (ADV and DYS). SE and SWS were also negatively correlated with LEDD in the DYS group. There was a negative correlation between dyskinesia severity and the estimated TST (eTST) over the 1-week actigraphy recording. In the early sleep, control subjects showed a significant greater amount of SWA, diffused over the whole scalp, compared to Parkinson’s disease patients. All groups, except the DYS group, manifested a clear-cut SWA decrease between early and late sleep.
Conclusions: Our data underline a strong pathophysiological association between sleep and Parkinson’s disease. Given that SWA may be a surrogate for synaptic strength, our data suggest that DYS patients do not have adequate synaptic downscaling. Further analysis is needed to determine the effect of drugs that can enhance cortical SWA in LIDs
References: Achermann P, Borbély AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience 1997; 81: 213-222. Agnew HW, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology 1966; 2: 263-266. Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003; 26: 342-392. Askenasy JJ, Yahr MD. Reversal of sleep disturbance in Parkinson's disease by antiparkinsonian therapy: a preliminary study. Neurology 1985; 35: 527-532. Bateman DE, Levett K, Marsden CD. Sleep benefit in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 1999; 67: 384-385. Bertolucci PH, Andrade LA, Lima JG, Carlini EA. Total sleep deprivation and Parkinson disease. Arq. Neuropsiquiatr. 1987; 45: 224-230. Brodsky MA, Park BS, Nutt JG. Effects of a dopamine agonist on the pharmacodynamics of levodopa in Parkinson disease. Arch. Neurol. 2010; 67: 27-32. Calabresi P, Filippo MD, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with Parkinson's disease: filling the bench-to-bedside gap. Lancet Neurol. 2010; 9: 1106-1117. Calabresi P, Pisani A, Rothwell J, Ghiglieri V, Obeso JA, Picconi B. Hyperkinetic disorders and loss of synaptic downscaling. Nat. Neurosci. 2016; 19: 868-875. Calandra-Buonaura G, Guaraldi P, Sambati L, Lopane G, Cecere A, Barletta G, et al. Multiple system atrophy with prolonged survival: is late onset of dysautonomia the clue? Neurol. Sci. 2013; 34: 1875-1878. Currie LJ, Bennett JP, Harrison MB, Trugman JM, Wooten GF. Clinical correlates of sleep benefit in Parkinson's disease. Neurology 1997; 48: 1115-1117. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004; 134: 9-21. Diederich NJ, Rufra O, Pieri V, Hipp G, Vaillant M. Lack of polysomnographic Non-REM sleep changes in early Parkinson's disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2013; 28: 1443-1446. Diederich NJ, Vaillant M, Mancuso G, Lyen P, Tiete J. Progressive sleep 'destructuring' in Parkinson's disease. A polysomnographic study in 46 patients. Sleep Med. 2005; 6: 313-318. Ferreira T, Prabhakar S, Kharbanda P. Sleep disturbances in drug naïve Parkinson′s disease (PD) patients and effect of levodopa on sleep. Ann. Indian Acad. Neurol. 2014; 17: 416. Fietze I, Quispe-Bravo S, Hänsch T, Röttig J, Baumann G, Witt C. Arousals and sleep stages in patients with obstructive sleep apnoea syndrome: Changes under nCPAP treatment. J. Sleep Res. 1997; 6: 128-133. Galati S, Salvade A, Pace M, Sarasso S, Baracchi F, Bassetti CL, et al. Evidence of an association between sleep and levodopa-induced dyskinesia in an animal model of Parkinson's disease. Neurobiol. Aging 2015; 36: 1577-1589. Galati S, Sarasso S, Moeller C, Kalein-Lang A, Staedler C. Synaptic homeostasis in Parkinson's disease: An high-density Eeg study in different stage of the disease. Mov. Disord. 2016; 31: S276. Galati S, Stefani A. Deep brain stimulation of the subthalamic nucleus: All that glitters isn't gold? Mov. Disord. Off. J. Mov. Disord. Soc. 2015; 30: 632-637. Galati S, Wei S, Orban G, Luft AR, Kaelin-Lang A. Cortical slow wave activity correlates with striatal synaptic strength in normal but not in Parkinsonian rats. Exp. Neurol. 2017. German DC, Manaye K, Smith WK, Woodward DJ, Saper CB. Midbrain dopaminergic cell loss in Parkinson's disease: computer visualization. Ann. Neurol. 1989; 26: 507-514. Gibb WR, Lees AJ. The significance of the Lewy body in the diagnosis of idiopathic Parkinson's disease. Neuropathol. Appl. Neurobiol. 1989; 15: 27-44. van Gilst MM, Louter M, Baumann CR, Bloem BR, Overeem S. Sleep benefit in Parkinson's disease: time to revive an enigma? J. Park. Dis. 2012; 2: 167-170. Goetz CG, Damier P, Hicking C, Laska E, Muller T, Olanow CW, et al. Sarizotan as a treatment for dyskinesias in Parkinson's disease: a double-blind placebo-controlled trial. Mov. Disord. Off. J. Mov. Disord. Soc. 2007; 22: 179-186. Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov. Disord. Off. J. Mov. Disord. Soc. 2007; 22: 41-47. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology 1998; 50: 318 and 16 pages following. Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J. Neurosci. Off. J. Soc. Neurosci. 2011; 31: 2481-2487. Huang Y-Z, Rothwell JC, Lu C-S, Chuang W-L, Chen R-S. Abnormal bidirectional plasticity-like effects in Parkinson's disease. Brain J. Neurol. 2011; 134: 2312-2320. Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature 2004; 430: 78-81. Kingsbury AE, Bandopadhyay R, Silveira-Moriyama L, Ayling H, Kallis C, Sterlacci W, et al. Brain stem pathology in Parkinson's disease: an evaluation of the Braak staging model. Mov. Disord. Off. J. Mov. Disord. Soc. 2010; 25: 2508-2515. Ku S, Glass GA. Age of Parkinson's disease onset as a predictor for the development of dyskinesia. Mov. Disord. Off. J. Mov. Disord. Soc. 2010; 25: 1177-1182. Linazasoro G. New ideas on the origin of L-dopa-induced dyskinesias: age, genes and neural plasticity. Trends Pharmacol. Sci. 2005; 26: 391-397. Lunardi G, Galati S, Tropepi D, Moschella V, Brusa L, Pierantozzi M, et al. Correlation between changes in CSF dopamine turnover and development of dyskinesia in Parkinson's disease. Parkinsonism Relat. Disord. 2009; 15: 383-389. Molina-Luna K, Pekanovic A, Röhrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti M-S, et al. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PloS One 2009; 4: e7082. Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson's disease and levodopa-induced dyskinesias. Brain J. Neurol. 2006; 129: 1059-1069. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002; 15: 1-25. Pascual-Marqui RD, Esslen M, Kochi K, Lehmann D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Methods Find. Exp. Clin. Pharmacol. 2002; 24 Suppl C: 91-95. Peeraully T, Yong M-H, Chokroverty S, Tan E-K. Sleep and Parkinson's disease: a review of case-control polysomnography studies. Mov. Disord. Off. J. Mov. Disord. Soc. 2012; 27: 1729-1737. Picconi B, Centonze D, Håkansson K, Bernardi G, Greengard P, Fisone G, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia [Internet]. Nat. Neurosci. 2003[cited 2015 Oct 12] Available from: http://www.nature.com/doifinder/10.1038/nn1040. Poe GR. Sleep Is for Forgetting. J. Neurosci. 2017; 37: 464-473. Rajan R, Popa T, Quartarone A, Ghilardi MF, Kishore A. Cortical plasticity and levodopa-induced dyskinesias in Parkinson's disease: Connecting the dots in a multicomponent network. Clin. Neurophysiol. 2017; 128: 992-999. Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, et al. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep 2007; 30: 1643-1657. Sara SJ. Sleep to Remember. J. Neurosci. 2017; 37: 457-463. Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson's disease. A community-based study. Brain J. Neurol. 2000; 123 ( Pt 11): 2297-2305. Sherif E, Valko PO, Overeem S, Baumann CR. Sleep benefit in Parkinson's disease is associated with short sleep times. Parkinsonism Relat. Disord. 2014; 20: 116-118. Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Stuttgart ; New York: Georg Thieme; 1988. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2010; 25: 2649-2653. Tononi G, Cirelli C. Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron 2014; 81: 12-34. Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004; 5: 97-107. de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 2017; 355: 507-510. Voon V, Napier TC, Frank MJ, Sgambato-Faure V, Grace AA, Rodriguez-Oroz M, et al. Impulse control disorders and levodopa-induced dyskinesias in Parkinson's disease: an update. Lancet Neurol. 2017; 16: 238-250. Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, et al. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson's disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2013; 28: 1064-1071. Yong M-H, Fook-Chong S, Pavanni R, Lim L-L, Tan E-K. Case control polysomnographic studies of sleep disorders in Parkinson's disease. PloS One 2011; 6: e22511.
To cite this abstract in AMA style:
N. Amato, M. Manconi, J.C. Möller, S. Sarasso, P. Stanzione, C. Städler, A. Kaelin-Lang, S. Galati. Levodopa-induced dyskinesia in Parkinson’s disease: Sleep matters [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/levodopa-induced-dyskinesia-in-parkinsons-disease-sleep-matters/. Accessed April 20, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/levodopa-induced-dyskinesia-in-parkinsons-disease-sleep-matters/