Objective: The objectives of this chart study were to evaluate adaptations in medication and flow rates and adverse events in patients with Parkinson’s disease (PD) before and after switch to levodopa-entacapone-carbidopa intestinal gel (LECIG) treatment.
Background: Until the introduction of LECIG, levodopa-carbidopa intestinal gel (LCIG) treatment was the only device-aided therapy available to improve response fluctuations in PD by means of continuous intestinal infusion of levodopa via a portable, programmable pump. The addition of entacapone and consequently reduction of a clinically sufficient daily dose of levodopa enabled the design of a smaller and lighter device. However, the number of trials that demonstrated the efficacy and tolerability of LCIG exceeds the small number of studies on LECIG.
Method: For a period of 18 months the charts of all 26 PD patients that started with LECIG in the Alrijne Hospital after September 2022 were screened. Every three months flow rate characteristics, the use of other anti-Parkinsonian drugs and the presence of adverse events were analyzed. Twelve patients switched from LCIG to LECIG. The period of 18 months before LECIG was initiated was also evaluated with a review of charts every 3 months until the onset of LECIG. For both LCIG and LECIG Friedman test was used to analyze changes in daytime infusion rates.
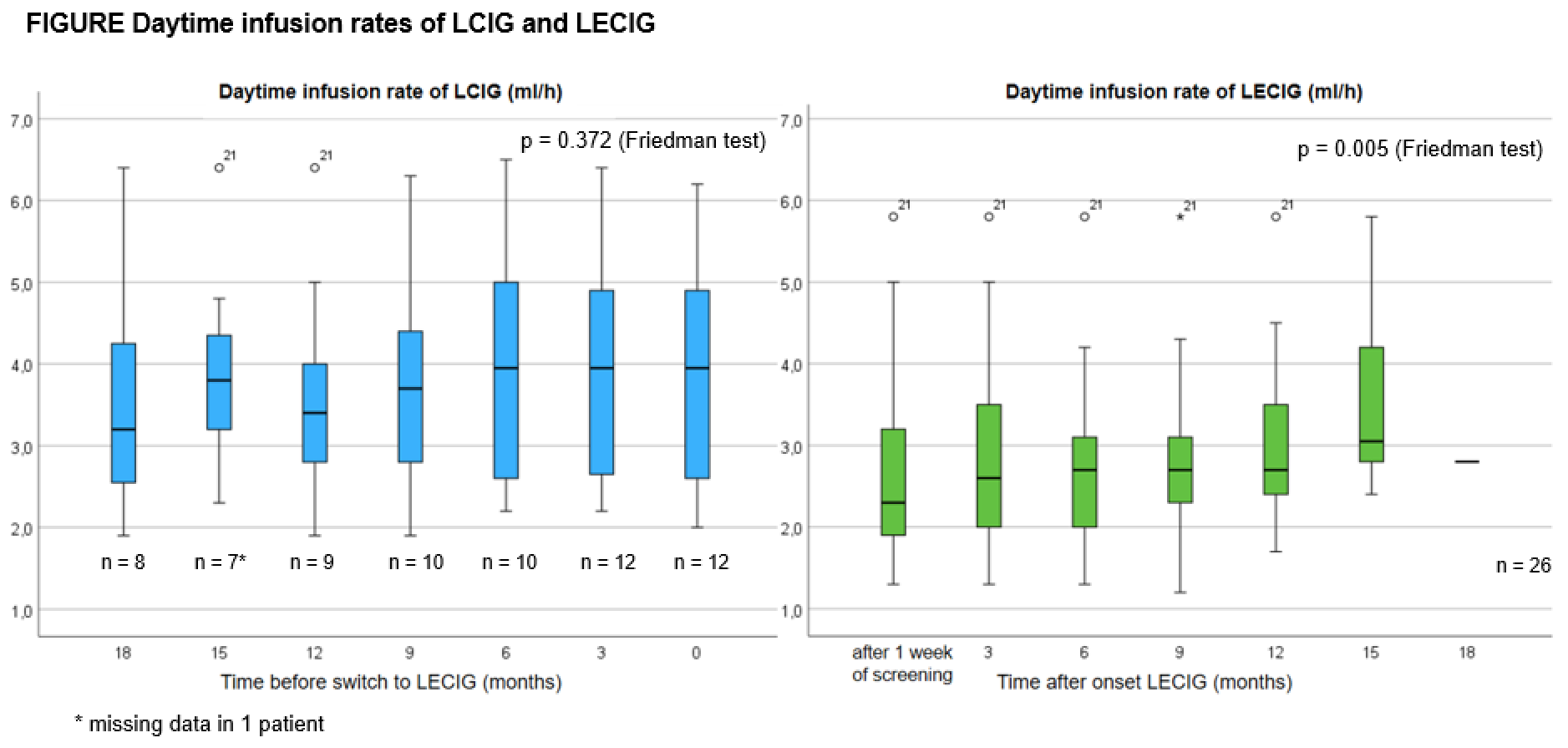
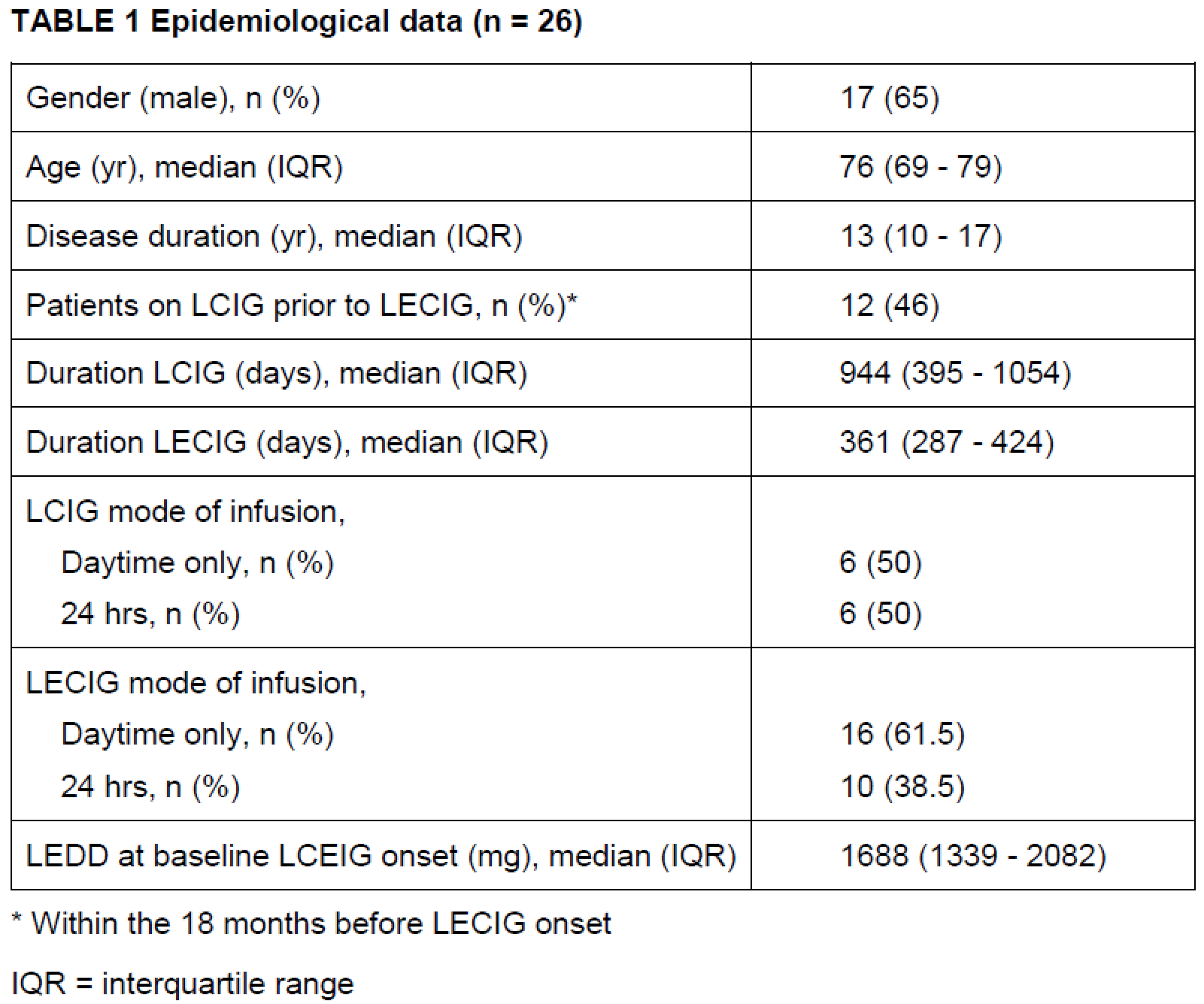
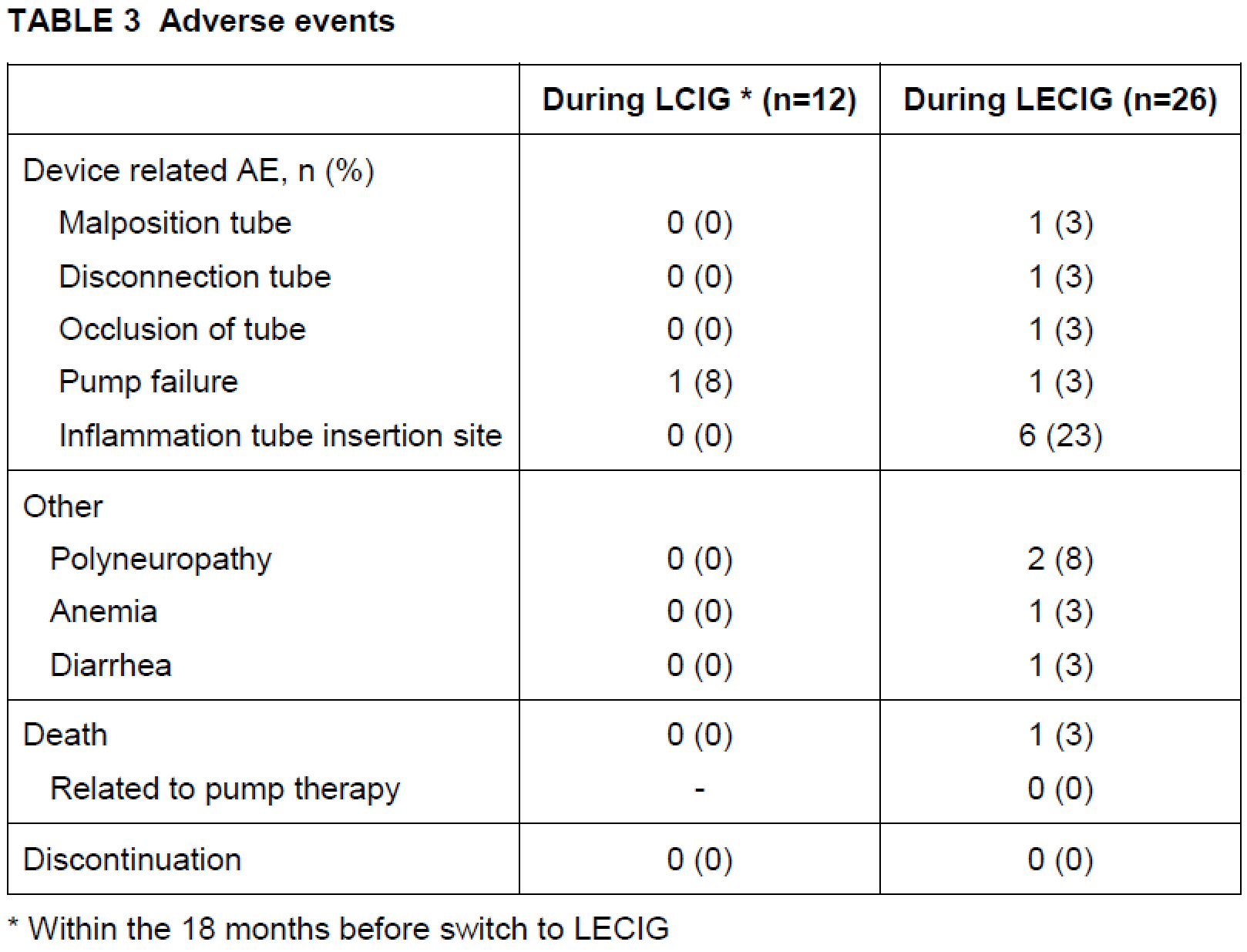
Results: The median (interquartile range [IQR]) age of PD patients included was 76 (69-79) years with a median (IQR) disease duration of 13 (10-17) years. The median (IQR) duration of LCIG and LECIG infusion was 944 (395-1054) and 361 (287-424) days respectively. Friedman test demonstrated significant increase of daytime infusion rates in the course of the disease for LECIG (p<0.05) but not for LCIG. Adverse events, including device-related complications were more frequently observed within the LECIG group without any discontinuation.
Conclusion: The infusion rate of LECIG gradually had to be increased over a period of 18 months or less, whereas day time infusion rates of LCIG remained relatively stable until LECIG was initiated. This may be the result of the onset of LECIG in a later stage of the disease and related to progression of symptoms in the course of the disease. Future research is warranted to compare LCIG and LECIG within similar stages of the disease, including the early stage.
Figure
Table 1
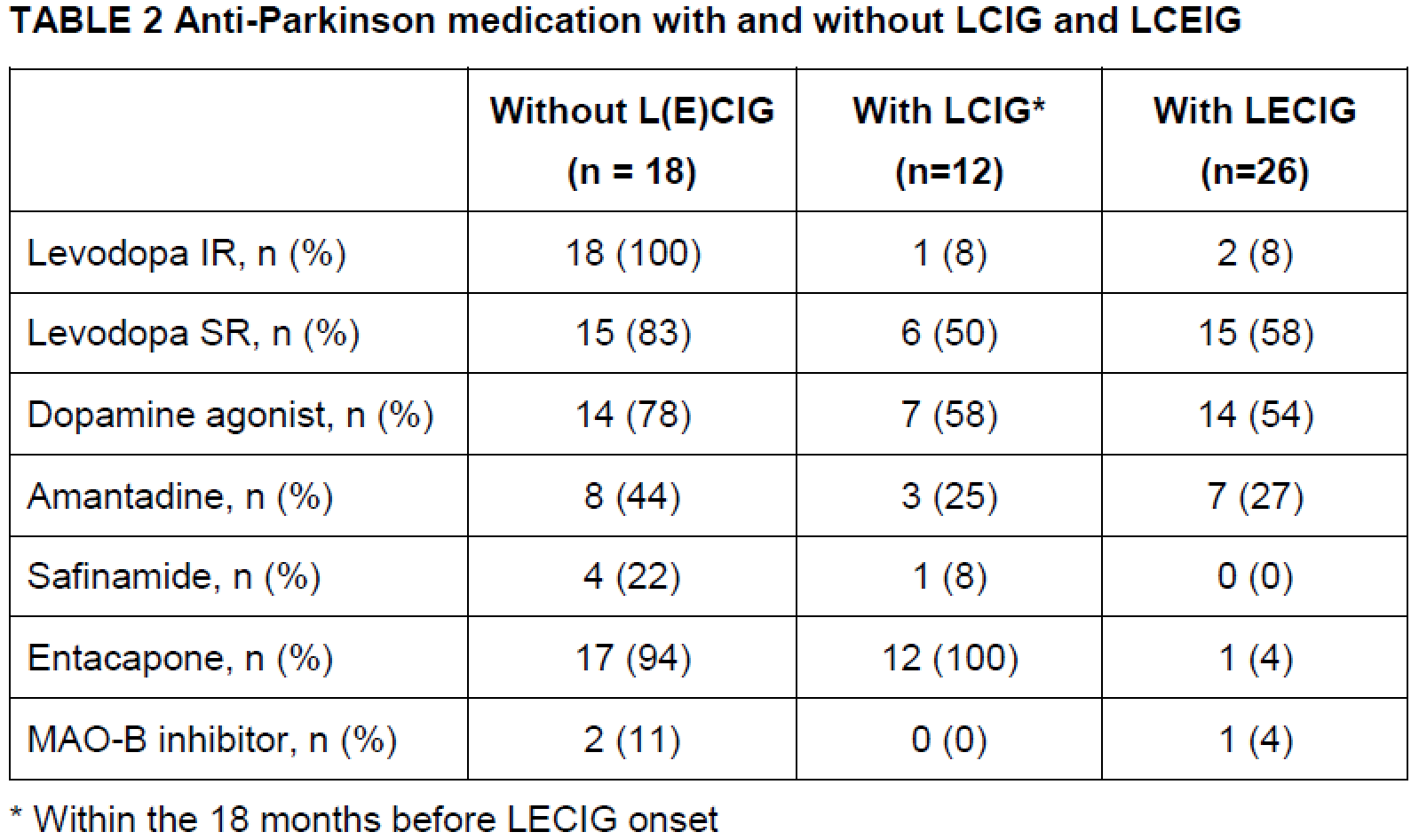
Table 2
Table 3
References: 1: Viljaharju V, Mertsalmi T, Pauls KAM, Koivu M, Eerola-Rautio J, Udd M,
Pekkonen E. Levodopa-Entacapone-Carbidopa Intestinal Gel Treatment in Advanced
Parkinson’s Disease: A Single-Center Study of 30 Patients. Mov Disord Clin
Pract. 2024 Feb;11(2):159-165.
2: Nyholm D, Jost WH. Levodopa-entacapone-carbidopa intestinal gel infusion in
advanced Parkinson’s disease: real-world experience and practical guidance. Ther
Adv Neurol Disord. 2022 Jun 26;15:17562864221108018.
3: Senek M, Nielsen EI, Nyholm D. Levodopa-entacapone-carbidopa intestinal gel
in Parkinson’s disease: A randomized crossover study. Mov Disord. 2017
Feb;32(2):283-286.
4: Antonini A, D’Onofrio V, Guerra A. Current and novel infusion therapies for
patients with Parkinson’s disease. J Neural Transm (Vienna). 2023
Nov;130(11):1349-1358.
To cite this abstract in AMA style:
AA. Vanderplas. Levodopa-Entacapone-Carbidopa Intestinal Gel Treatment In Parkinson’s Disease: A Single-Center Retrospective Chart Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/levodopa-entacapone-carbidopa-intestinal-gel-treatment-in-parkinsons-disease-a-single-center-retrospective-chart-study/. Accessed December 20, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/levodopa-entacapone-carbidopa-intestinal-gel-treatment-in-parkinsons-disease-a-single-center-retrospective-chart-study/