Session Information
Date: Thursday, June 23, 2016
Session Title: Parkinson's disease: Clinical trials, pharmacology and treatment
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To evaluate the tolerability and effect of levodopa-carbidopa intestinal gel (LCIG, designated in the US as carbidopa-levodopa enteral suspension) on non-motor symptoms (NMS), quality of life (QoL) in advanced Parkinson’s disease (PD) patients during 24 months (M) of routine care.
Background: LCIG is continuously delivered via percutaneous gastrojejunostomy (PEG-J) in advanced PD patients with motor fluctuations and dyskinesia not adequately reduced by available oral anti-Parkinsonian medications. LCIG treatment significantly improved NMS and quality of life in this registry at interim (12M). (ref 1)
Methods: In this multinational (18 countries, 75 centres) registry, LCIG was titrated via nasojejunal (NJ) tube and delivered over 24M via PEG-J. The mean Non-motor Symptom Scale (NMSS) and 8-item Parkinson’s disease Questionnaire (PDQ-8) total scores were assessed. Adverse drug reactions (ADRs), which are adverse events with at least a possible relationship to treatment, were monitored from the titration phase until 28 days after the final visit.
Results: Of the 375 patients who enrolled, 258 (69%) completed the study. (Table 1) There was a significant decrease in the mean change from baseline on the NMSS and PDQ-8 total scores at every time point. (Figures 1 and 2) There were 194 patients (54%) who had an ADR and 109 (31%) who had a serious ADR. (Table 1) Twenty-five deaths occurred during the course of the study and 28-day follow-up.
| Disposition | n (% of N=375) |
| Enrolled | 375 (100) |
| Completed | 258 (69) |
| Prematurely discontinued, due to: | 117 (31) |
| Administrative | 7 (1.9) |
| Adverse drug reaction (ADR) | 46 (12) |
| Lack of efficacy | 11 (2.9) |
| Lost to follow-up | 16 (4.3) |
| Protocol violation | 5 (1.3) |
| Withdrew consent | 31 (8.3) |
| Missing reason | 1 (0.3) |
| Safety | n (% of N=356b) |
| Any ADR | 194 (54) |
| Any serious ADR | 109 (31) |
| Death | 25 (7.0) |
| ADRs occurring in ≥3% patientsa: | |
| Weight decreased | 24 (6.7) |
| Device related infection | 21 (5.9) |
| Device dislocation | 17 (4.8) |
| Device issue | 17 (4.8) |
| Polyneuropathy | 16 (4.5) |
| Device lead issue | 14 (3.9) |
| Medical device complication | 13 (3.7) |
| Abdominal pain | 13 (3.7) |
| Hallucination | 12 (3.4) |
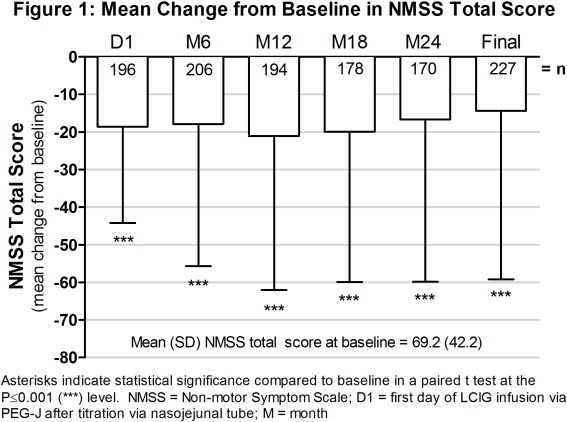
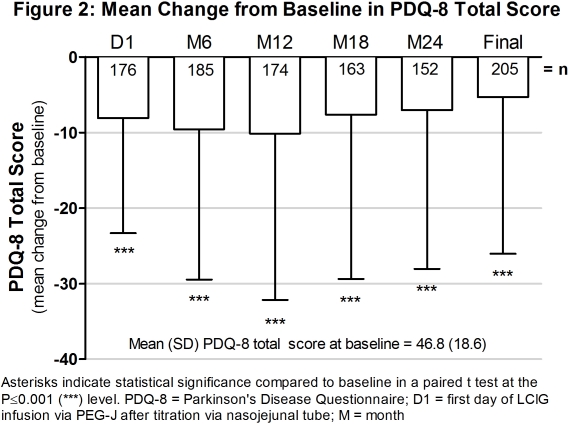
Conclusions: There were significant improvements in NMS and QoL in LCIG-treated advanced PD patients. The observed safety and tolerability was consistent with the established safety profile of LCIG. (ref 1 and 2). References: 1. Antonini et al. Parkinsonism Relat Disord. 2015; 21(3):231-5. 2. Fernandez et al. Mov Disord. 2015; 30(4):500-9.
To cite this abstract in AMA style:
K.R. Chaudhuri, Z. Pirtosek, B. Pickut, W. Poewe, F. Valldeoriola, L. Defebvre, R. Jech, P. Odin, C. Winkler, J. Szasz, K. Onuk, A. Yegin, L. Bergmann, A. Antonini. Levodopa-carbidopa intestinal gel in routine care of advanced Parkinson’s disease patients: Final long-term non-motor, quality of life and safety results from the GLORIA registry [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/levodopa-carbidopa-intestinal-gel-in-routine-care-of-advanced-parkinsons-disease-patients-final-long-term-non-motor-quality-of-life-and-safety-results-from-the-gloria-registry/. Accessed January 1, 2026.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/levodopa-carbidopa-intestinal-gel-in-routine-care-of-advanced-parkinsons-disease-patients-final-long-term-non-motor-quality-of-life-and-safety-results-from-the-gloria-registry/
