Objective: To review long-term real-world experience and clinical outcomes in PD patients with LCIG therapy over 11 years in a multidisciplinary University clinic setting.
Background: Levodopa Carbidopa Intestinal Gel (LCIG) therapy has been shown to be a safe and effective treatment for advanced Parkinson’s disease (PD). Limited data is available regarding long-term benefits and complications in Canada.
Method: This is an ambidirectional cohort study with retrospective chart review performed from 2011-2016 while data was collected prospectively from 2017 to 2022. Consecutive patients on LCIG therapy at our centre between 2011 and 2022 were included. Data collected: dosing, UPDRS-III motor scores, OFF times, hours with dyskinesias, MoCA, complications, discontinuation reasons and nursing time requirements.
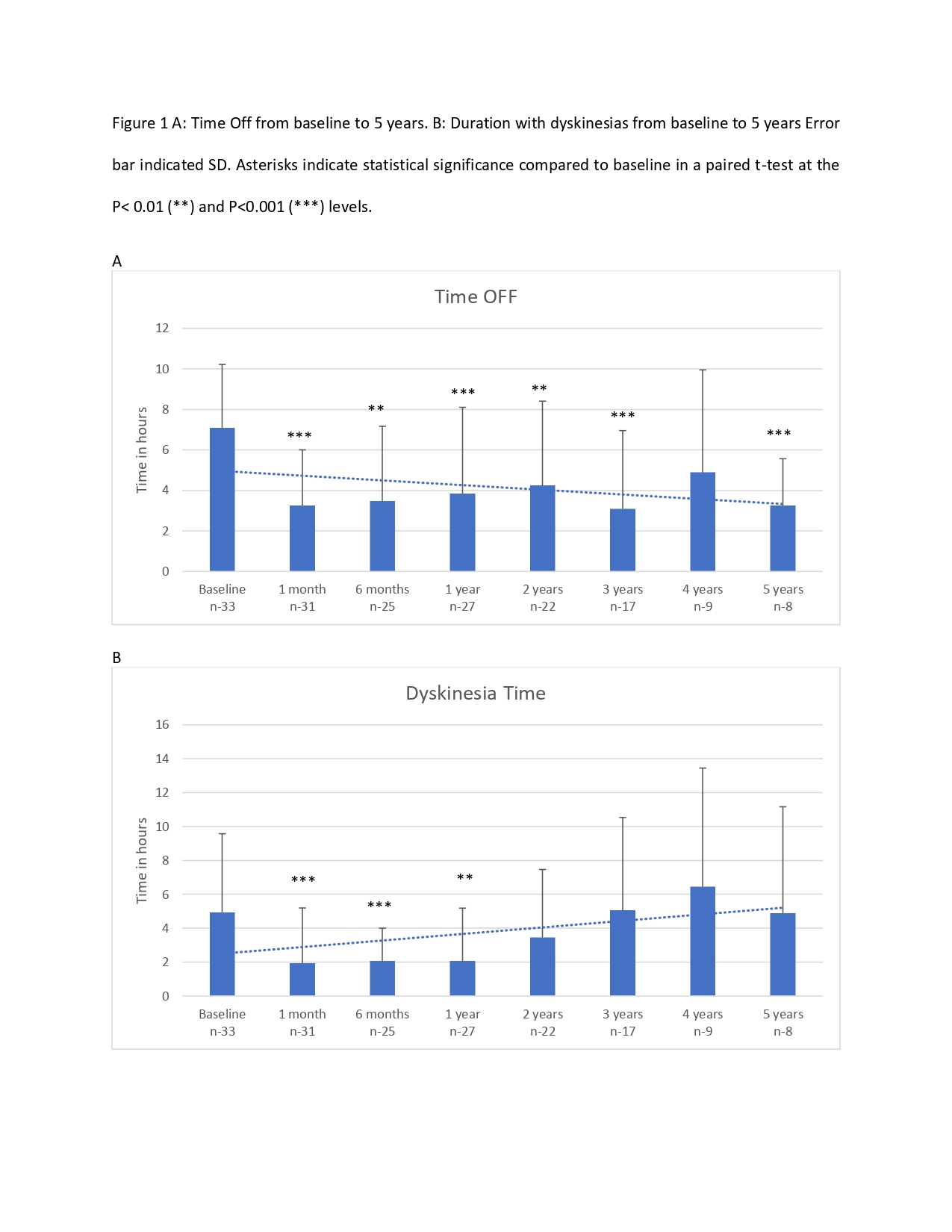
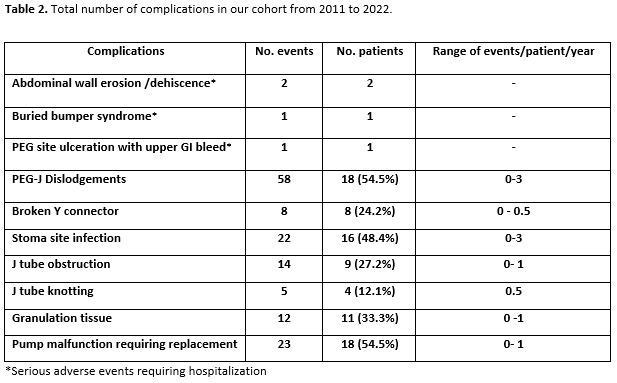
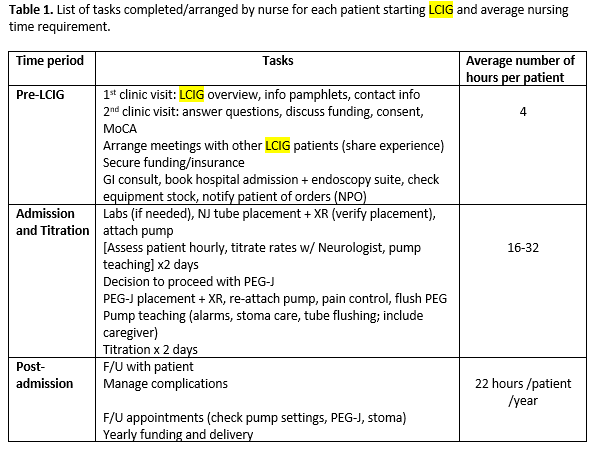
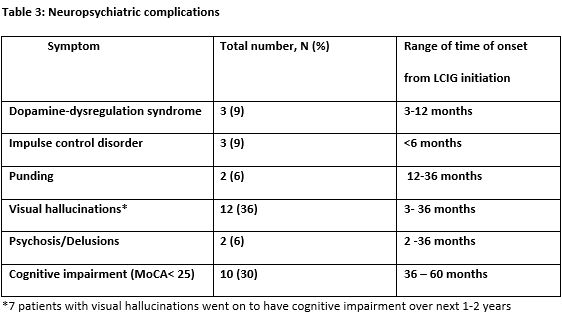
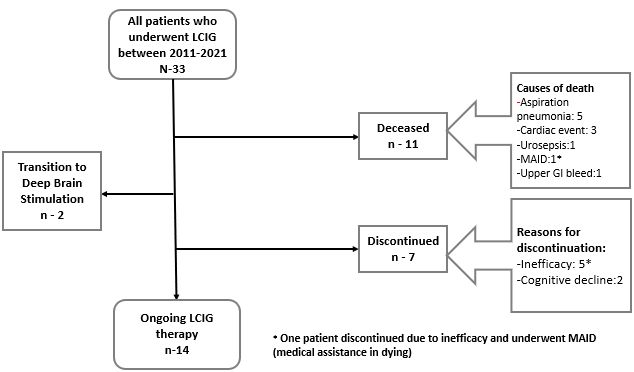
Results: Thirty-three patients received LCIG therapy with a mean follow up of 3.25+2.09 years. UPDRS-III scores showed reduction by 15% from baseline (mean 35.9) up to 4 years (mean 30.4). Daily OFF time improved from baseline (mean 7.1+3.13 hours) up to 5 years (mean 3.3+2.31 hours; -53.5%; p < 0.048) and dyskinesias remained stable [Figure 1]. Nursing time averaged 22 hours per patient per year after PEG-J insertion and titration [Table 1]. Most common complications were PEG-J tube dislodgement and stoma site infection (0 – 3 events/patient/year) [Table 2]. Serious side effects were seen in 4 (12%) patients resulting in hospitalization and /or death. The most neuropsychiatric complication was visual hallucinations seen in 12 (36%) patients developing over 1-3 years after LCIG initiation [Table 3]. Of these, cognitive impairment occurred in 7 patients over 3-5 years. Over the study period, 9 patients (27%) discontinued the treatment including 7 patients due to lack of efficacy (2 of these transitioned to DBS) and 2 patients due to worsening of cognition and 10 (30%) patients died of causes unrelated to LCIG infusion [Figure 2].
Conclusion: Our study confirmed long-term efficacy of LCIG in treatment of advanced PD as evidenced by improvement in motor scores, reduced OFF-times and stable dyskinesias over 5-year follow-up. Overall satisfaction rate in 70% of patients was high. Complications are frequent but generally mild and necessitates a well trained multi-disciplinary team for management.
To cite this abstract in AMA style:
C. Vekhande, M. Hamed, G. Tremain, J. Mah, A. Shetty, A. Lazarescu, O. Suchowersky. Levodopa Carbidopa Intestinal Gel for Parkinson’s Disease over 11 years: A Single Center “Real-World” Experience [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/levodopa-carbidopa-intestinal-gel-for-parkinsons-disease-over-11-years-a-single-center-real-world-experience/. Accessed December 20, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/levodopa-carbidopa-intestinal-gel-for-parkinsons-disease-over-11-years-a-single-center-real-world-experience/