Category: Parkinson's Disease: Neuroimaging
Objective: We performed a systematic meta-analysis of SN iron imaging studies in PD to better understand the role of iron-sensitive MRI quantification to distinguish patients from healthy controls. We also studied the factors that may influence iron quantification and analyzed the correlations between demographic and clinical data and iron load.
Background: Parkinson’s disease (PD) is a progressive neurodegenerative disease whose main neuropathological feature is the loss of dopaminergic neurons of the substantia nigra (SN) (1). There is also an increase in iron content in the SN in postmortem (2,3) and imaging studies using iron-sensitive MRI techniques (4-6). However, MRI results are variable across studies (7-13).
Method: We searched Pubmed and Science-direct databases (from January 1994 to December 2019) for studies that analyzed iron load in the SN of PD patients using T2*, R2*, susceptibility weighting imaging (SWI) or quantitative susceptibility mapping (QSM) and compared the values with healthy controls. Details for each study regarding participants, imaging methods, and results were extracted. The effect size and confidence interval of 95% were calculated for each study as well as the weighted effect size for each marker over studies. Hence, the correlations between technical and clinical metrics with iron load were analyzed.
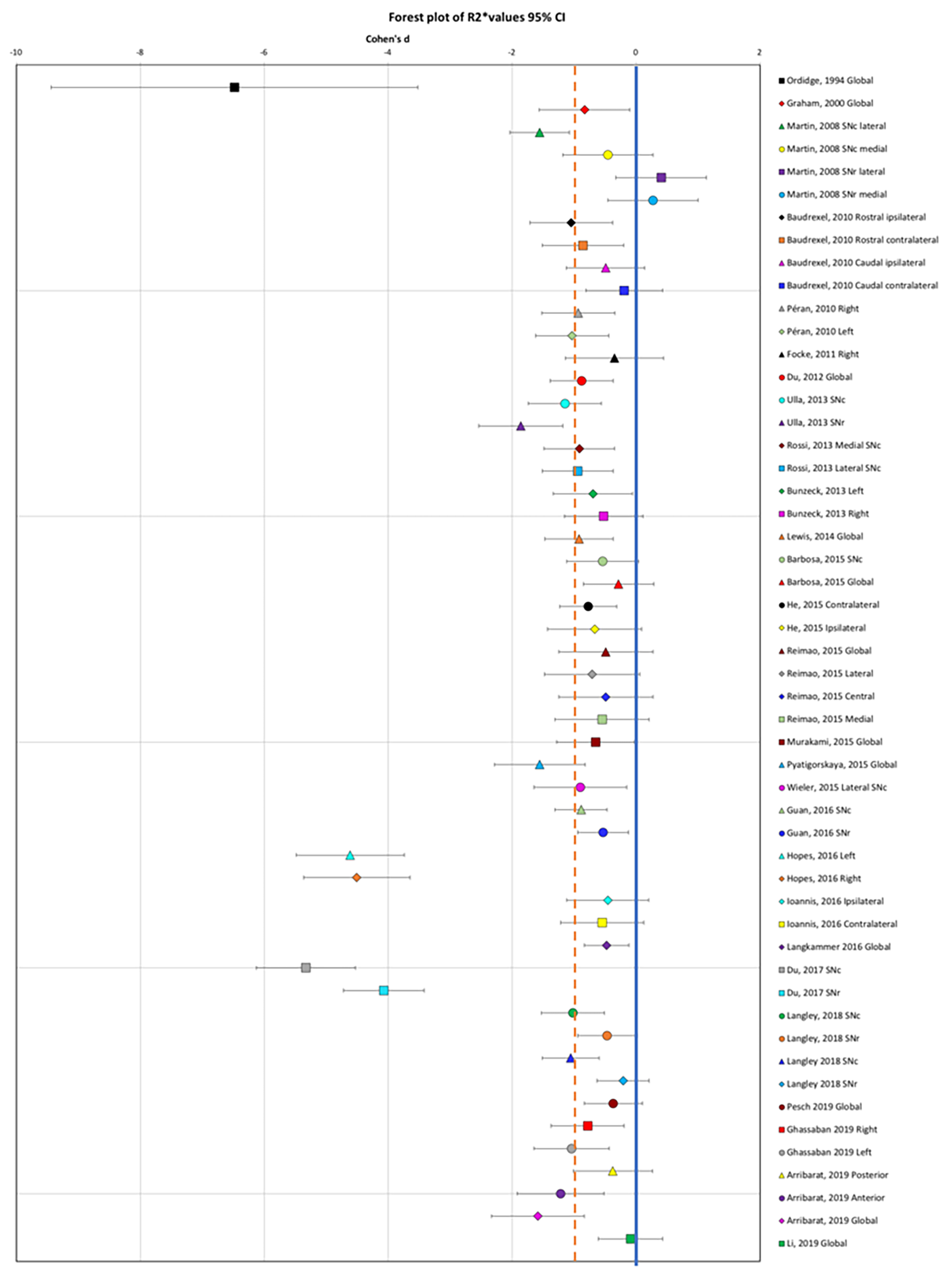
Results: Forty-six articles fulfilled the inclusion criteria including 27 for T2*/R2* measures, 10 for SWI, and 17 for QSM (3135 patients and 1675 controls). Eight of the articles analyzed both R2* and QSM. A notable effect size was found in the SN in PD for R2* increase (effect size: -1.08, 95% confidence interval (CI): -1.77 to -0.40,) [figure1], for SWI measurements (1.13, 95% CI: 0.01 to 1.16) and for QSM increase (-1.44, 95% CI -2.04 to -0.83). Correlations between imaging measures and UPDRS scores were mostly observed for QSM.
Conclusion: The consistent increase in MRI measures of iron content in PD across the literature using R2*, SWI or QSM techniques, confirmed that these measurements provided reliable markers of iron content in PD. Several of these measurements correlated with the severity of motor symptoms. Lastly, QSM appeared more robust and reproducible than R2* and more suited to multicenter studies
References: 1. Fearnley JM, Lees AJ. Brain (1991) 114 ( Pt 5):2283–2301. 2. Langkammer C, Krebs N, Goessler W et al. Radiology (2010) 257:455–462. 3. Langkammer C, Schweser F, Krebs N et al. Neuroimage (2012) 62:1593–1599. 4. Pyatigorskaya N, Sharman M, Corvol J-C et al. Mov Disord (2015) 30:1077–1084. 5. Graham JM, Paley MN, Grünewald RA,et al. Brain (2000) 123 Pt 12:2423–2431. 6. Hopes L, Grolez G, Moreau C et al. PLoS ONE (2016) 11:e0147947. 7. Pyatigorskaya N, Sharman M, Corvol J-C et al. Mov Disord (2015) 30:1077–1084. 8. Graham JM, Paley MN, Grünewald RA et al. Brain (2000) 123 Pt 12:2423–2431. 9. Hopes L, Grolez G, Moreau C et al. PLoS ONE (2016) 11:e0147947. 10. Arribarat G, Pasternak O, De Barros A et al. Parkinsonism Relat Disord (2019) 65:146–152. 11. Isaias IU, Trujillo P, Summers P et al. Front Aging Neurosci (2016) 8 12. Langley J, He N, Huddleston DE et al. Mov Disord (2019) 34:416–419. 13. Li G, Zhai G, Zhao X, An H et al. Neuroimage (2019) 188:465–472.
To cite this abstract in AMA style:
N. Pyatigorskaya, C. Sanz Morere, R. Gaurav, E. Biondetti, R. Valabregue, M. Santin, L. Yahia-Cherif, S. Lehericy. Iron Imaging as a Diagnostic Tool for Parkinson’s Disease: A Systematic Review and Meta-Analysis [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/iron-imaging-as-a-diagnostic-tool-for-parkinsons-disease-a-systematic-review-and-meta-analysis/. Accessed April 26, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/iron-imaging-as-a-diagnostic-tool-for-parkinsons-disease-a-systematic-review-and-meta-analysis/