Session Information
Date: Sunday, October 7, 2018
Session Title: Spasticity
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: •To use real-world registry data to augment available safety data, product performance and support overall efficacy. •To utilize registry data to broaden awareness of this therapeutic option in the treatment of severe spasticity.
Background: ITB therapy has demonstrated efficacy in the treatment of spinal and cerebral associated severe spasticity with more than 25 years of clinical experience. Access to this therapy, however, remains limited due to questions related to the safety of implantable infusion systems required for therapy delivery. Our intent is to use long-term prospective data collected under a global registry to assess product performance, therapy safety, and patient satisfaction.
Methods: A prospective, long-term, multi-center registry called the Product Surveillance Registry (PSR) enrolled patients, after providing informed consent, implanted with the SynchroMed® II infusion system. Data is collected on the performance of the pump and catheter. Patients are followed prospectively for events related to the device, procedure, and therapy. Investigators provide event descriptions, patient symptoms, and patient outcomes.
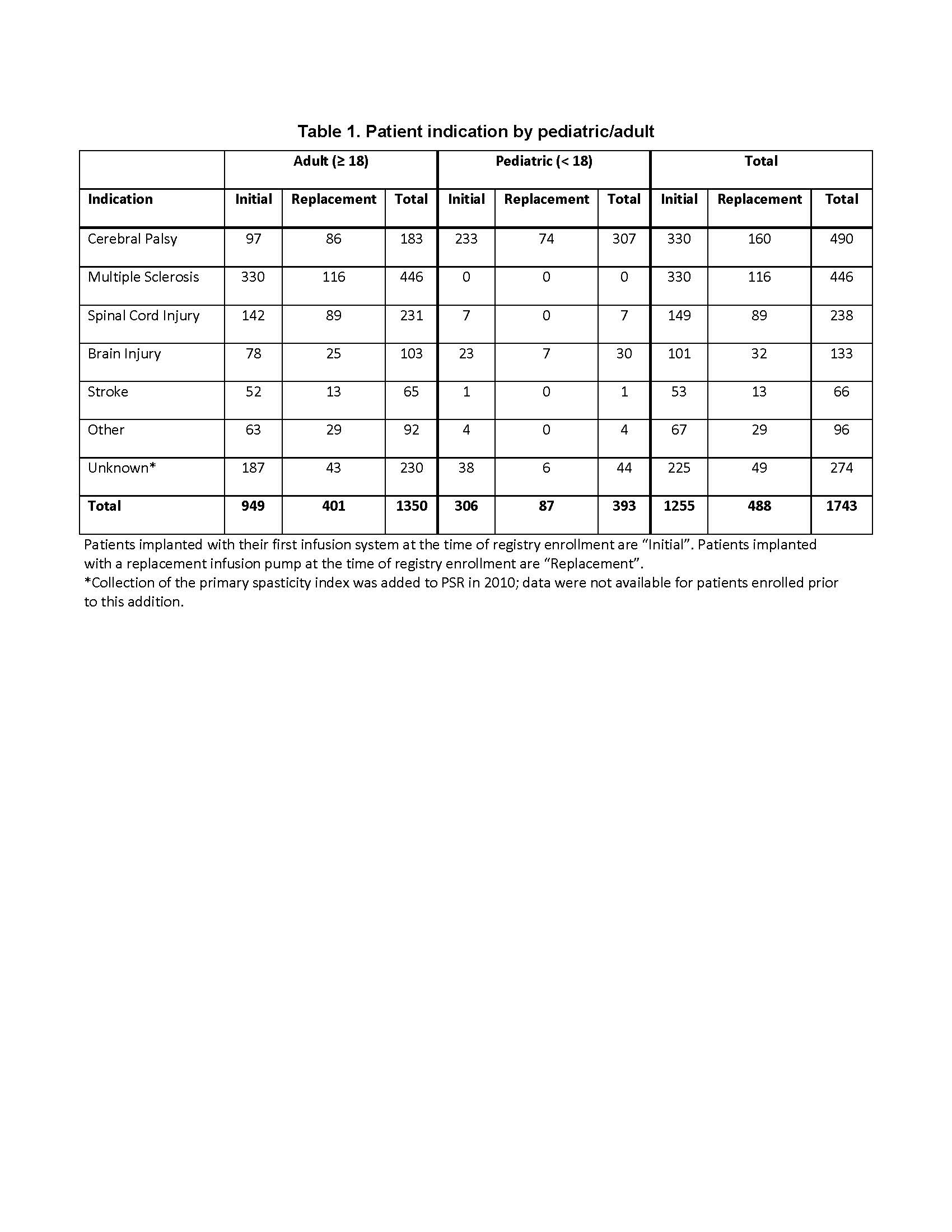
Results: A total of 1743 patients (77% adult, 53.2% male) being treated with ITB for severe spasticity (Table 1) have been followed at 53 registry sites between August 2003 and October 2017, for an accumulated 6,481 patient-years. Of PSR discontinuations, study exit due to an adverse event was limited to 0.3%, with the majority (58.6%) due to site closure and patient relocation. After 10 years, 87.2% of adult and 76.3% of pediatric patients continued with ITB in PSR. Overall, 98.9% of pumps reaching end of battery life were replaced at the time of explant.
Conclusions: Long-term ITB therapy for the treatment of severe spasticity requires surgical implant of a programmable infusion system. Complications with this implanted device may necessitate surgical intervention for correction. Within this registry across multiple spasticity origins and in both pediatrics and adults, low therapy discontinuation rates due to an adverse event were observed, as was high acceptance of surgical intervention for therapy continuation. The patient/caregiver willingness to accept surgical and continued therapy risks for therapy continuation, as a surrogate for overall patient satisfaction, was extremely high.
References: Deer TR, Prager J, Levy R et al. Polyanalgesic Consensus Conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation 2012;15:436–466.
To cite this abstract in AMA style:
M. Schiess, S. Eldabe, R. Spencer, K. Stromberg, T. Weaver, R. Plunkett. Intrathecal baclofen (ITB) therapy in the treatment of severe spasticity: Safety and Product Performance from a Large Multinational Multi-Center Registry [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/intrathecal-baclofen-itb-therapy-in-the-treatment-of-severe-spasticity-safety-and-product-performance-from-a-large-multinational-multi-center-registry/. Accessed October 22, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/intrathecal-baclofen-itb-therapy-in-the-treatment-of-severe-spasticity-safety-and-product-performance-from-a-large-multinational-multi-center-registry/