Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: The objective of this research work is to develop a lipid nanocarrier system of Bromocriptine and evaluate its increased efficacy in the management of Parkinson’s Disease (PD). The developed system is administered via the non-invasive nasal route for efficient transfer to the brain.
Background: Age-related Parkinson’s disease (PD) is the most common neurodegenerative motor disorder in the world, causing tremor, rigidity, bradykinesia and gait impairment (1). One serious problem in administration of neuro therapeutics is its inability to cross the Blood Brain Barrier (BBB) and show its efficacy (2). Hence, novel nano formulations are administered via the nasal route for rapid and selective targeting to the brain by transport via the olfactory pathway bypassing the BBB and avoiding peripheral distribution and associated potential adverse effects.
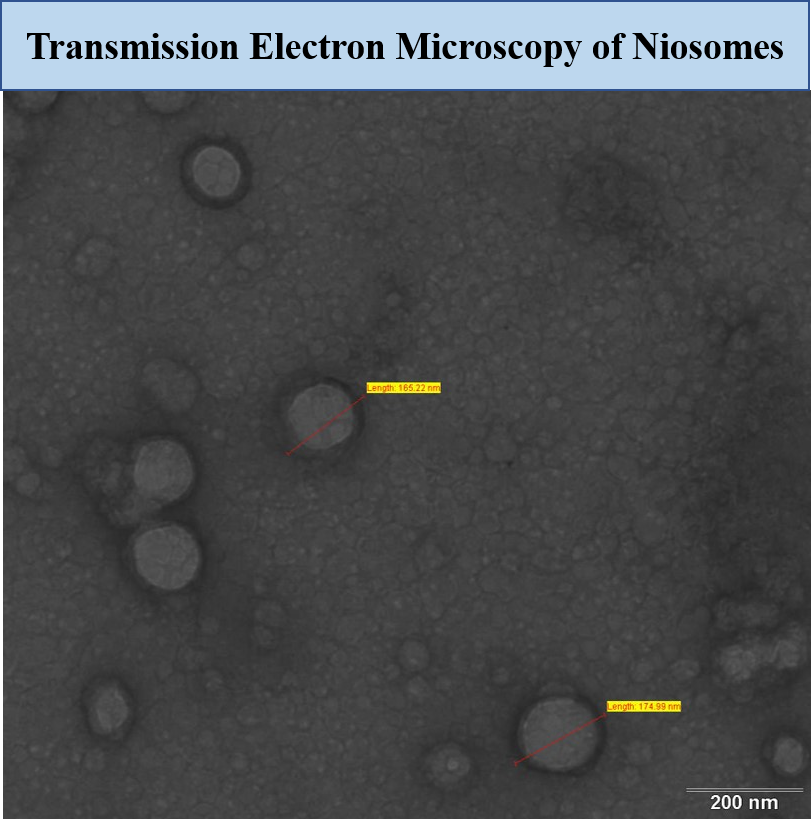
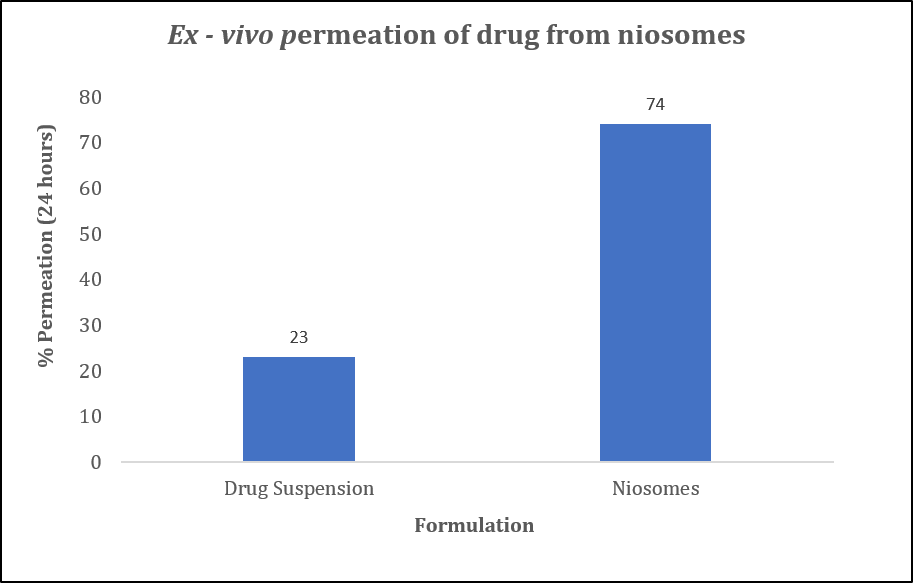
Methods: Stable surfactant vesicles i.e niosomes were prepared by ethanol injection method. The optimized vesicular formulation was evaluated for its size, surface morphology by Transmission Electron Microscopy (TEM), % drug encapsulation and haemocompatability studies. Niosomes were evaluated for drug permeation across freshly excised goat nasal mucosa to understand the % enhancement in permeation as compared to plain drug solution. Cataleptic activity of the developed system was evaluated in a haloperidol induced rat model to establish enhanced efficacy of niosomes over plain drug suspension.
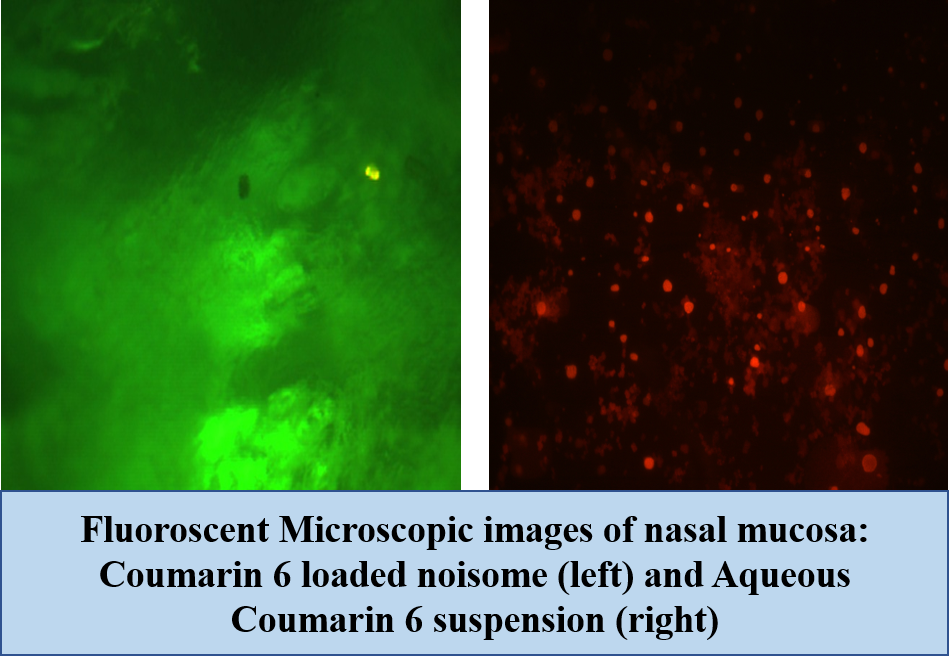
Results: Niosomes were found to be spherical in shape measuring 175.32 nm (figure 1). The encapsulation of drug was found to be 60 %. Niosomes were found to be hemo compatible. Ex – vivo permeation studies on goat nasal mucosa revealed nearly 3.2 times enhancement in permeation as compared to plain drug suspension (figure 2). This can be corroborated by fluorescent microscopic images (figure 3) of coumarin-6 loaded niosomes across nasal mucosa as compared to coumarin-6 aqueous suspension. Histopathological examinations of nasal mucosa showed no signs of necrosis and hemorrhage. Haloperidol induced rat models showed a marked improvement in reversal of catalepsy behavior treated with intranasal niosomes as compared to intranasal plain drug suspension.
Conclusions: Results from various studies suggest that niosomes are safe and effective delivery systems for targeted delivery of Bromocriptine following the direct nose to brain pathway. The composition of the vesicles facilitated enhanced transport of the drug across nasal mucosa. This nano-carrier system not only serves as a non-invasive therapeutic alternative in the management of PD but also is a platform technology that can be extrapolated to numerous other drugs to target the brain.
References: 1. Complexity of dopamine metabolism, Johannes Meiser, Daniel Weindl and Karsten Hiller, Meiser et al. Cell Communication and Signaling 2013, http://www.biosignaling.com/content/11/1/34. 2. Leonard AK, Sileno AP, Brandt GC, Foerder CA, Quay SC, Costantino HR. In vitro formulation optimization of intranasal galantamine leading to enhanced bioavailability and reduced emetic response in vivo. Int J Pharm. 2007;335(1-2):138-146.
To cite this abstract in AMA style:
S. V G, P. Vavia. Intranasal Delivery of Bromocriptine loaded Lipid Nano Carriers for Enhanced Brain Delivery in Parkinson’s disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/intranasal-delivery-of-bromocriptine-loaded-lipid-nano-carriers-for-enhanced-brain-delivery-in-parkinsons-disease/. Accessed January 8, 2026.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/intranasal-delivery-of-bromocriptine-loaded-lipid-nano-carriers-for-enhanced-brain-delivery-in-parkinsons-disease/