Category: Parkinson’s Disease: Clinical Trials
Objective: Characterize inter- and intra-day variability of β-glucocerebrosidase (GCase) activity, using a live-cell assay, and glucosyl-β sphingosine levels in healthy volunteers (HVs) and Parkinson’s disease (PD) patients with a GBA1 mutation (GBA-PD).
Background: Heterozygous mutations in the gene GBA1, which result in reduced GCase enzyme activity, are a major risk factor for PD. Current methods of measuring GCase activity do not account for the physiology of the lysosomal environment. This study used a novel, live-cell assay to conduct real-time assessments of GCase activity in blood samples from HVs and GBA-PD patients to evaluate the potential for this assay to measure target engagement of GCase activators.
Method: Whole blood was obtained from 8 HVs and 12 GBA-PD patients at 3 time points (Day 1, 0 and 4 h, and Day 8, time matched to 0 h). GCase activity was evaluated using the GCase substrate PFB-FDGlu in whole blood, followed by flow cytometry analysis to assess activity in monocytes. GCase activity was also assessed in dried blood spots (DBS) from the same samples. Mean GCase activity and plasma glucosyl-β sphingosine concentrations were analyzed.
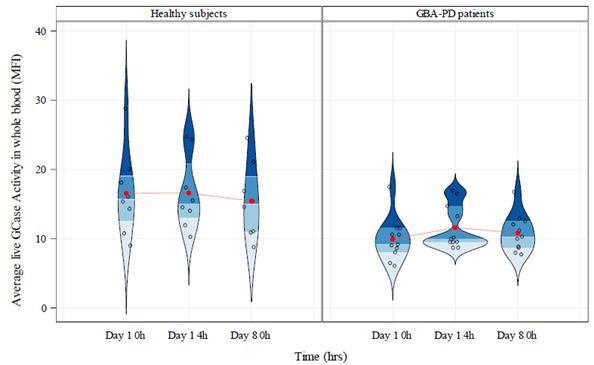
Results: GCase activity was 33.4% lower in GBA-PD patients than in HVs, p=0.0044 [figure 1]. Inter-day coefficient of variation (CV) within subjects was 24.0% in HVs and 37.1% in GBA-PD patients. Intra-day variability within subjects for GCase activity was 16.8% in HVs and 25.9% in GBA-PD patients. Although not statistically significant in this small cohort, plasma glucosyl-β sphingosine concentrations were higher in GBA-PD patients than in HVs (0.31 pmol/mL (95% CI: -0.014, 0.64), p=0.0595). Inter- and intra-day CVs in glucosyl-β sphingosine within subjects were 2.4% and 0% in HVs, and 1.7% and 0% in GBA-PD patients, respectively. Using DBS, mean GCase activity was lower in GBA-PD patients than HVs, but was not statistically significant.
Conclusion: Using a novel live-cell GCase activity assay in whole blood, we observed biochemically relevant reductions in GCase activity in GBA-PD patients compared to HVs with minimal intra- and inter-day variability. In this study, this assay demonstrated a greater discriminative potential compared to DBS. These results support the use of this assay to assess target engagement of GCase activators in clinical trials.
Violin graph GCase activity in whole blood (MFI)
To cite this abstract in AMA style:
J. Vandervalk, D. Dumas, E. Thijssen, D. Pereira, W. Grievink, Y. Yavuz, C. Pesch, D. Ysselstein, M. Hagey, J. Cedarbaum, K. Hunt, P. Kremer. Inter- and intra-day variability in β-Glucocerebrosidase activity and pathway biomarkers in healthy volunteers and patients with Parkinson’s disease with a GBA1 mutation [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/inter-and-intra-day-variability-in-%ce%b2-glucocerebrosidase-activity-and-pathway-biomarkers-in-healthy-volunteers-and-patients-with-parkinsons-disease-with-a-gba1-mutation/. Accessed December 24, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/inter-and-intra-day-variability-in-%ce%b2-glucocerebrosidase-activity-and-pathway-biomarkers-in-healthy-volunteers-and-patients-with-parkinsons-disease-with-a-gba1-mutation/