Category: Parkinson’s Disease: Clinical Trials
Objective: To describe the safety profile and tolerability of BIA-28-6156 observed during phase 1 clinical trials.
Background: BIA-28-6156 is a novel allosteric activator of beta-glucocerebrosidase (GCase) that is being developed for the proposed indication of treatment of Parkinson’s disease (PD) in patients with a mutation in the β-glucocerebrosidase (GBA1) gene (GBA-PD). Heterozygous mutations in GBA1 are recognized as the most frequent PD genetic risk.[1]
Method: This was a post-hoc analysis of safety data from the completed phase 1 clinical trials in healthy subjects and GBA-PD patients. A total of 9 studies with BIA-28-6156 in single and multiple doses, ranging from 3mg to 150mg, were included. Adverse events were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) 25.1 version and assessed by causality and seriousness. Treatment emergent adverse events (TEAEs) were tabulated and summarized according to System Organ Class (SOC) and preferred terms (PT) within each SOC.
Results: Cumulative clinical safety data from 327 subjects (264 BIA-28-6156; 63 Placebo) was collected. 216 (66,1%) subjects were male, and average age was 47,8 years (±16,9). Among the subjects who received BIA 28-6156, 222 (84,1%) were healthy subjects and 42 (15,9%) were GBA-PD patients. Most of these (134; 50,8%) were exposed to a dose of 60 mg of BIA-28-6156.
In the overall safety population, a total of 170 (52,0%) subjects reported at least one TEAE; 48 (18,2%) subjects reported at least one event considered to be related to BIA-28-6156.
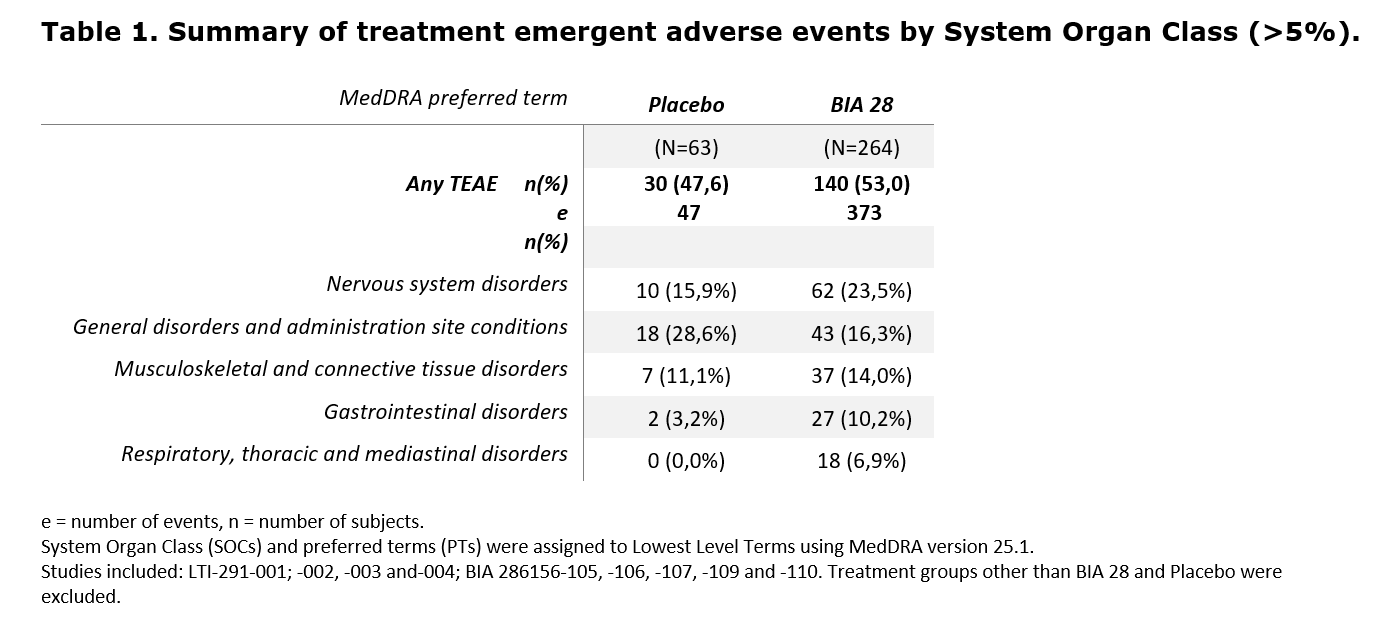
The SOC Nervous System Disorders was the most representative (BIA-28-6156: 23,5% vs. Placebo: 15,9%), followed by General disorders and administration site conditions (16,3% vs. 28,6%), and Musculoskeletal and connective tissue disorders (14,0% vs. 11,1%)[table 1].
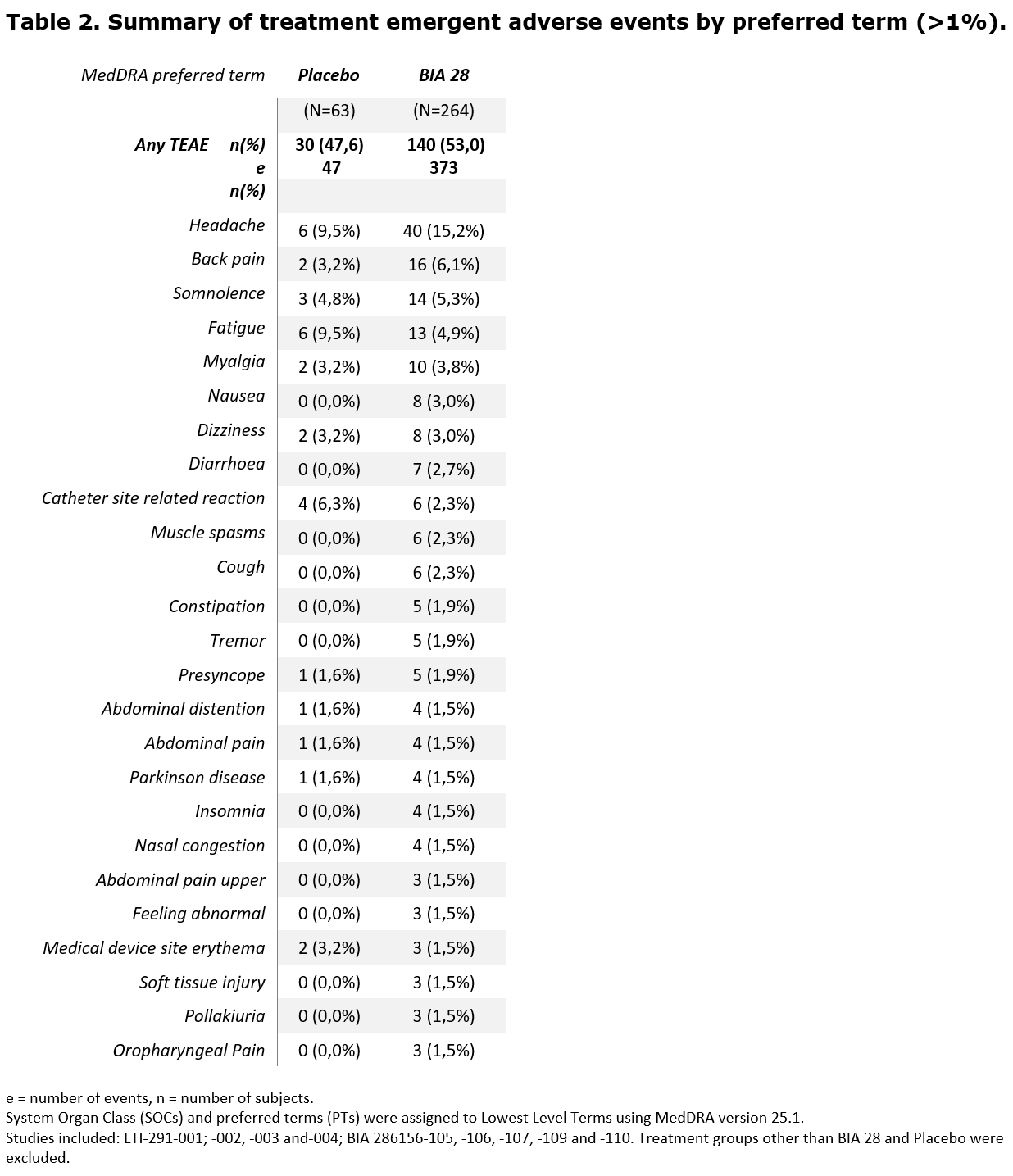
The most frequently reported TEAEs (>3%) were headache (BIA-28-6156: 15,2% vs. Placebo: 9,5%), back pain (6,1% vs. 3,2%), somnolence (5,3% vs. 4,8%), fatigue (4,9% vs. 9,5%) and myalgia (3,8% vs. 3,2%)[table 2].
All TEAEs with BIA-28-6156 were mild in severity except for 14 moderate events (two considered related: “abdominal pain” and “hepatobiliary disease”). No serious TEAE was identified.
Conclusion: BIA-28-6156 was well-tolerated and presented a favorable safety profile in healthy subjects and in GBA-PD patients. No relevant safety concerns were identified in completed phase I clinical studies.
References: [1] Siebert M, Sidransky E, Westbroek W. Glucocerebrosidase is shaking up the synucleinopathies. Brain 2014;137(pt 5):1304–1322.
To cite this abstract in AMA style:
L. Guedes, D. Hilt, M. Fonseca, I. Peixoto, H. Gama. Integrated safety analysis of BIA-28-6156 phase 1 clinical trials (a novel allosteric activator of beta-glucocerebrosidase) [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/integrated-safety-analysis-of-bia-28-6156-phase-1-clinical-trials-a-novel-allosteric-activator-of-beta-glucocerebrosidase/. Accessed January 1, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/integrated-safety-analysis-of-bia-28-6156-phase-1-clinical-trials-a-novel-allosteric-activator-of-beta-glucocerebrosidase/