Category: Parkinson’s Disease: Clinical Trials
Objective: To characterize reported infusion site infection adverse events (AEs) and their clinical management in patients with Parkinson’s disease (PD) treated with foslevodopa/foscarbidopa (LDP/CDP).
Background: LDP/CDP is a formulation of levodopa/carbidopa prodrugs delivered as a continuous (24-hour/day) subcutaneous infusion. Phase 3 studies indicate LDP/CDP has a favorable benefit-risk profile, with systemic safety generally consistent with that of oral LD/CD. Infusion site reactions (ISRs) were the most common AEs. As with other subcutaneous therapies, ISRs may reflect localized inflammatory reactions although infusion site infections are also reported. The majority of such events were non-serious, mild to moderate in severity and resolved.
Method: This integrated post-hoc analysis summarizes AEs reported by investigators as presumed infusion site infection under the terms infection, cellulitis, and/or abscess in patients with advanced PD who received LDP/CDP during phase 3 clinical trials. Four trials were included: a 12-week, phase 3, randomized, double-blind trial (NCT04380142); a 52-week, phase 3, open-label, single-arm trial (NCT03781167); and their 2 open-label extension studies (NCT04379050 and NCT04750226).
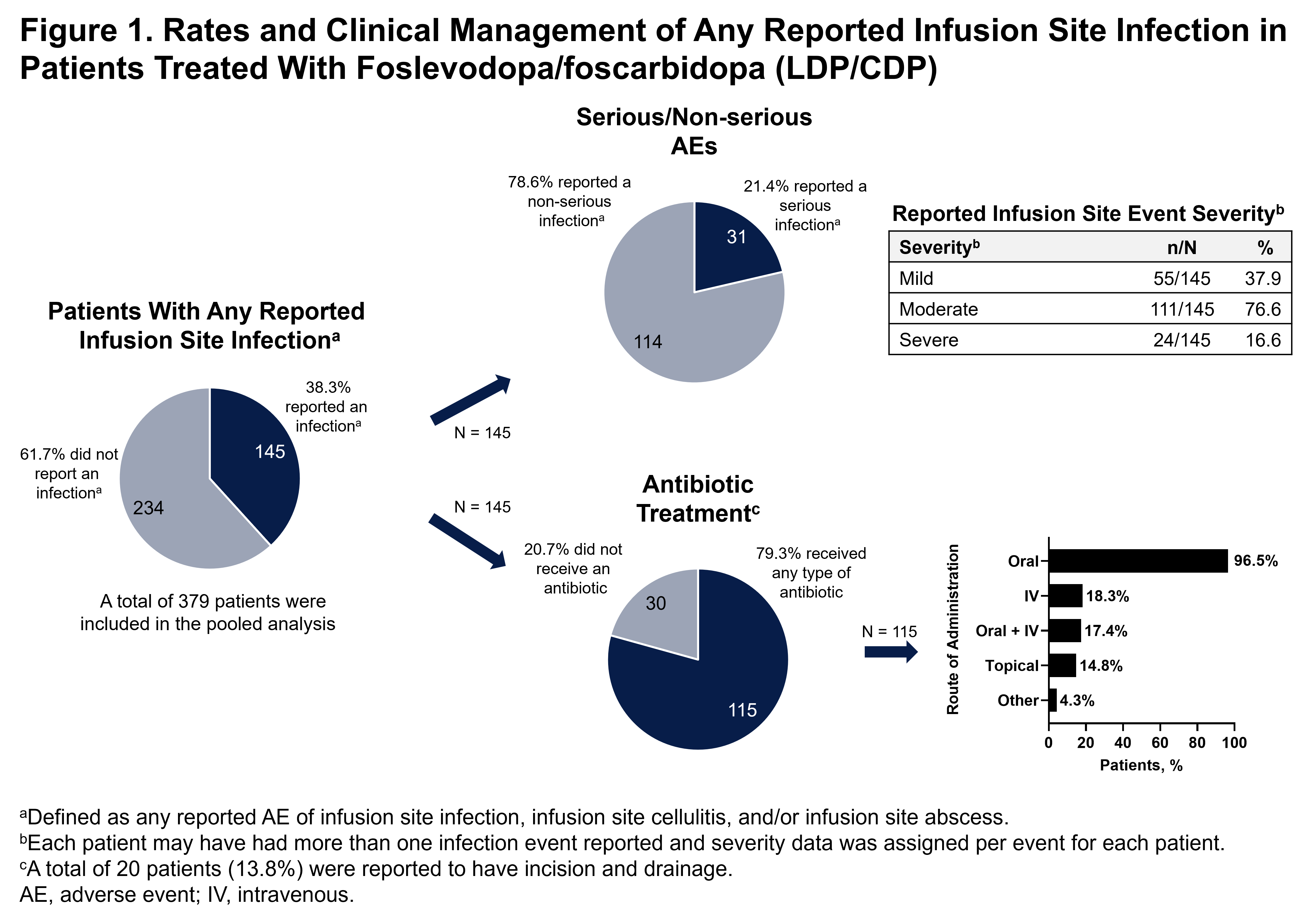
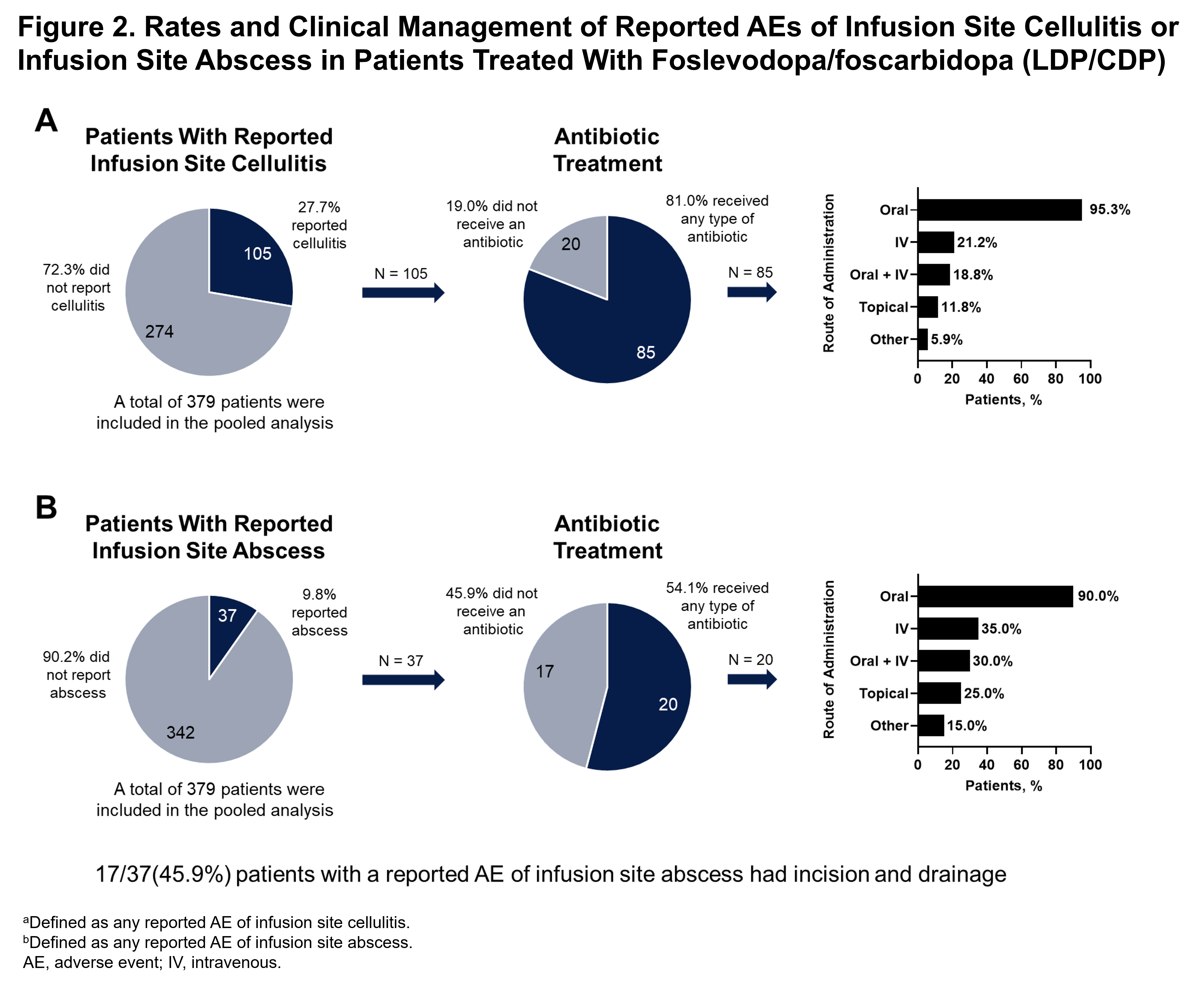
Results: 379 patients were treated with LDP/CDP. Investigators reported AEs of infusion site infection, cellulitis, and/or abscess in 145 (38.3%) patients; the majority were reported as non-serious, mild to moderate in severity and resolved [figure1]. Despite clinically presumed infection, infusion site culture was infrequently performed and uncommonly identified any bacterial pathogen. Of patients with reported infusion site infection, cellulitis, and/or abscess, 115 (79.3%) received primarily empiric antibiotic therapy [figure1] and 20 (13.8%) had incision and drainage [figure 2]. Only 24 patients (16.6%) with reported infections discontinued treatment with LDP/CDP due to the events.
Conclusion: Although infusion site events were the most common AEs reported in patients who received LDP/CDP, the majority were non-serious, mild to moderate in severity and resolved. Most presumed infusion site infections were managed with empiric oral antibiotics and did not lead to discontinuation of therapy.
To cite this abstract in AMA style:
P. Odin, T. Kimber, B. Bergmans, E. Freire-Alvarez, F. Gandor, S. Isaacson, S. Dhanani, R. Hauser, M. O'Meara, A. Jeong, J. Jia, R. Gupta, L. Bergmann, M. Shah, L. Harmer, S. Talapala, J. Aldred. Integrated analysis of phase 3 trials of foslevodopa/foscarbidopa demonstrated that majority of reported infusion site AEs including infections were non serious, mild to moderate in severity and did not result in treatment discontinuation. [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/integrated-analysis-of-phase-3-trials-of-foslevodopa-foscarbidopa-demonstrated-that-majority-of-reported-infusion-site-aes-including-infections-were-non-serious-mild-to-moderate-in-severity-and-did-n/. Accessed December 19, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/integrated-analysis-of-phase-3-trials-of-foslevodopa-foscarbidopa-demonstrated-that-majority-of-reported-infusion-site-aes-including-infections-were-non-serious-mild-to-moderate-in-severity-and-did-n/