Session Information
Date: Tuesday, September 24, 2019
Session Title: Parkinsonisms and Parkinson-Plus
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: In this study, we employed vesicular monoamine transporter 2–overexpressing mice (VMAT2-HI) to investigate the influence of increased vesicular storage of catacholamines on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced impairment of neurogenesis.
Background: By sequestering DA into vesicle, VMAT2 maintains the low level of DA in neuronal cytosol and protects the neuron from damage [1-3]. Previous studies have shown that increased vesicular monoamine transporter 2 protected against MPTP neurotoxicity [4]. Since MPTP-induced impairment of neurogenesis was referred in some studies and was related to nonmotor symptoms in the context of PD [5, 6], increased vesicular monoamine transporter may oppose MPTP-related neurodegeneration via enhanced ability of neurogenesis.
Method: 2- month-old VMAT2-HI and WT littermates were investigated in the study and injected MPTP or saline 20mg/kg at 2-h intervals for four times within a single day. 24h after the last administration of MPTP, mice were treated with Brdu (50mg/Kg/day) for consequent five days. Mice were sacrificed at predetermined time points (7 and 28 d) after the last injection of MPTP. For proliferation experiments, mice were sacrificed on day 7 and Brdu-staining cells were counted. Meanwhile, TH expression in nigrostriatal system including substantia nigra, striatum were detected by westernblot and immunofluorescent staining. DA and its metabolites were analyzed via liquid chromatography with electrochemical detection. For the “survival” experiments, mice were sacrificed on day 28 and Brdu-labeled cells were detected.
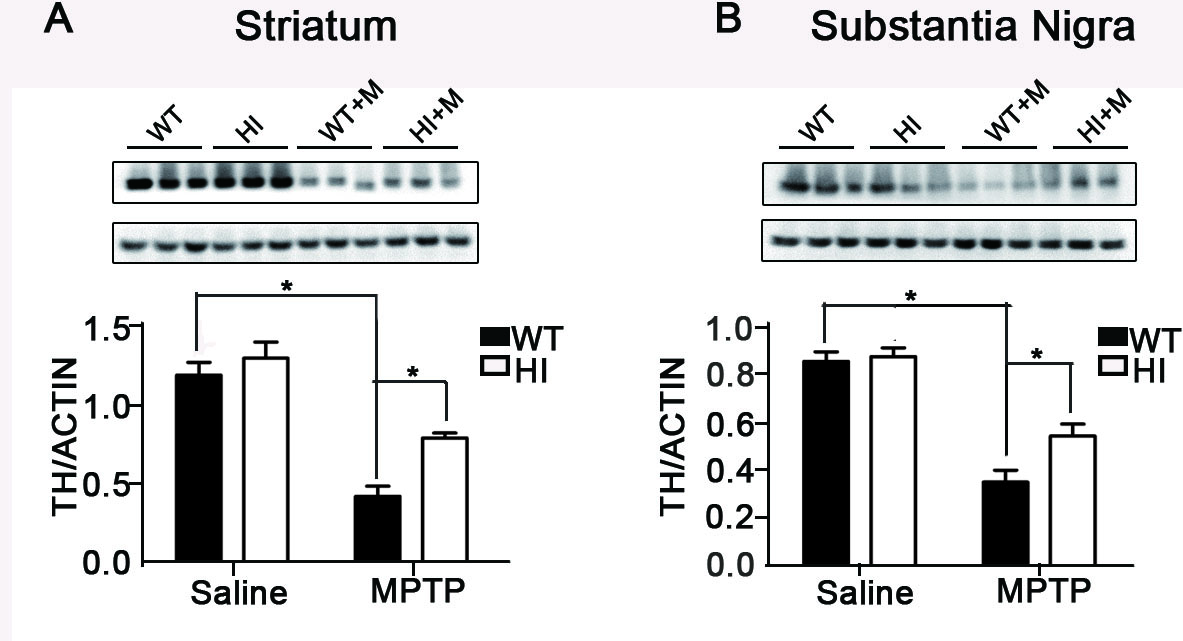
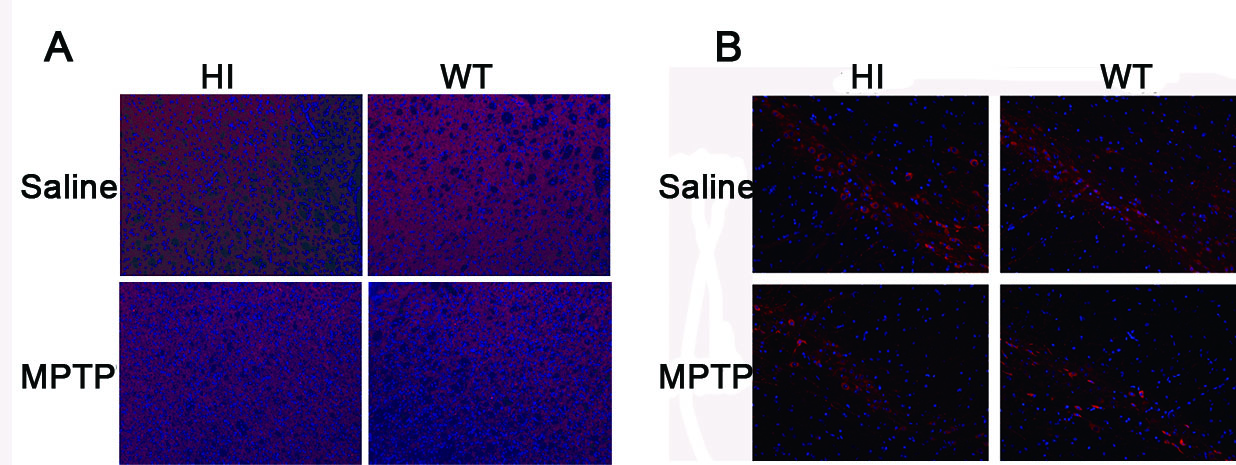
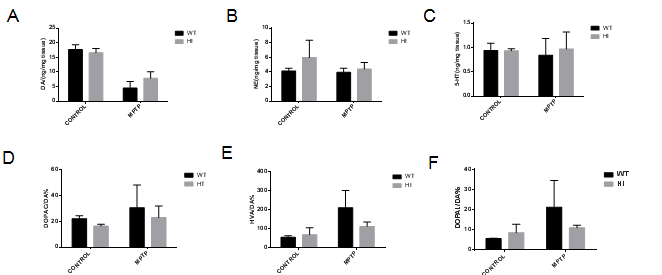
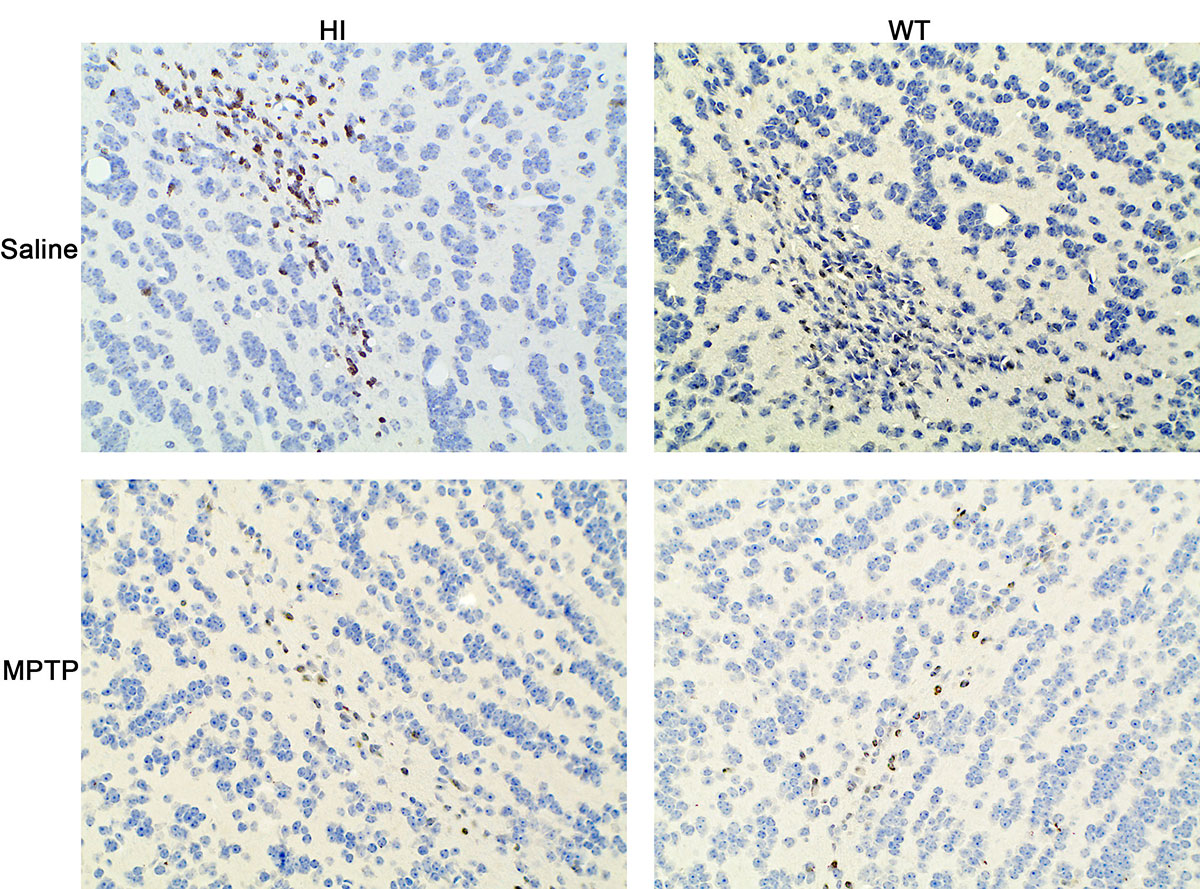
Results: VMAT2-HI mice show a significantly higher number of remaining TH+ neurons in the SNc compared to WT mice in response to MPTP (figure1 B). Administration of MPTP decreased expression of TH in the striatum of VMAT2-HI and WT mice, but more obvious in WT (figure1 A). TH immunohistochemistry staining confirmed the similar results (figure2). Analysis of HPLC results showed both the genotype exhibited an obvious decrease in DA content after exposure to MPTP and the amplitude was more prominent in VMAT2 WT mice compared to HI mice (figure3). Basal neurogenesis was identical between the two genotypes at the age of 2 months. MPTP-treated VMAT2 HI mice displayed more Brdu+ cell compared to MPTP-treated WT mice (figure4).
Conclusion: Our data demonstrated that VMAT2-HI mice alleviate MPTP-induced impairment of neurogenesis.
References: [1] Erickson J.D., Eiden L.E. Functional identification and molecular cloning of a human brain vesicle monoamine transporter [J]. Journal of neurochemistry, 1993, 61(issue):2314-2317. [2] Liu Y., Roghani A., Edwards R.H. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium [J]. Proc. Natl. Acad. Sci. U. S. A., 1992, 89(issue):9074-9078. [3] Erickson J.D., Eiden L.E., Hoffman B.J. Expression cloning of a reserpine-sensitive vesicular monoamine transporter [J]. Proc. Natl. Acad. Sci. U. S. A., 1992, 89(issue):10993-10997. [4] Lohr K.M., Bernstein A.I., Stout K.A., et al. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo [J]. Proc. Natl. Acad. Sci. U. S. A., 2014, 111(issue):9977-9982. doi:10.1073/pnas.1402134111. [5] He X.J., Nakayama H. Transiently impaired neurogenesis in MPTP mouse model of Parkinson’s disease [J]. Neurotoxicology, 2015, 50(issue):46-55. doi:10.1016/j.neuro.2015.07.007. [6] Zhang T., Hong J., Di T., et al. MPTP Impairs Dopamine D1 Receptor-Mediated Survival of Newborn Neurons in Ventral Hippocampus to Cause Depressive-Like Behaviors in Adult Mice [J]. Frontiers in molecular neuroscience, 2016, 9(issue):101. doi:10.3389/fnmol.2016.00101.
To cite this abstract in AMA style:
K. Ma, G. Zhang, C. Han, X. Guo, Y. Xia, F. Wan, L. Kou, S. Yin, L. Liu, N. Xiong, J. Huang, T. Wang. Increased vesicular monoamine transporter alleviate MPTP-induced impairment of neurogenesis [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/increased-vesicular-monoamine-transporter-alleviate-mptp-induced-impairment-of-neurogenesis/. Accessed April 26, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/increased-vesicular-monoamine-transporter-alleviate-mptp-induced-impairment-of-neurogenesis/