Objective: To summarize clinical experience with incobotulinumtoxinA (INCO) for treating chronic sialorrhea due to Parkinson’s disease (PD), atypical parkinsonism, stroke, or traumatic brain injury in adults.
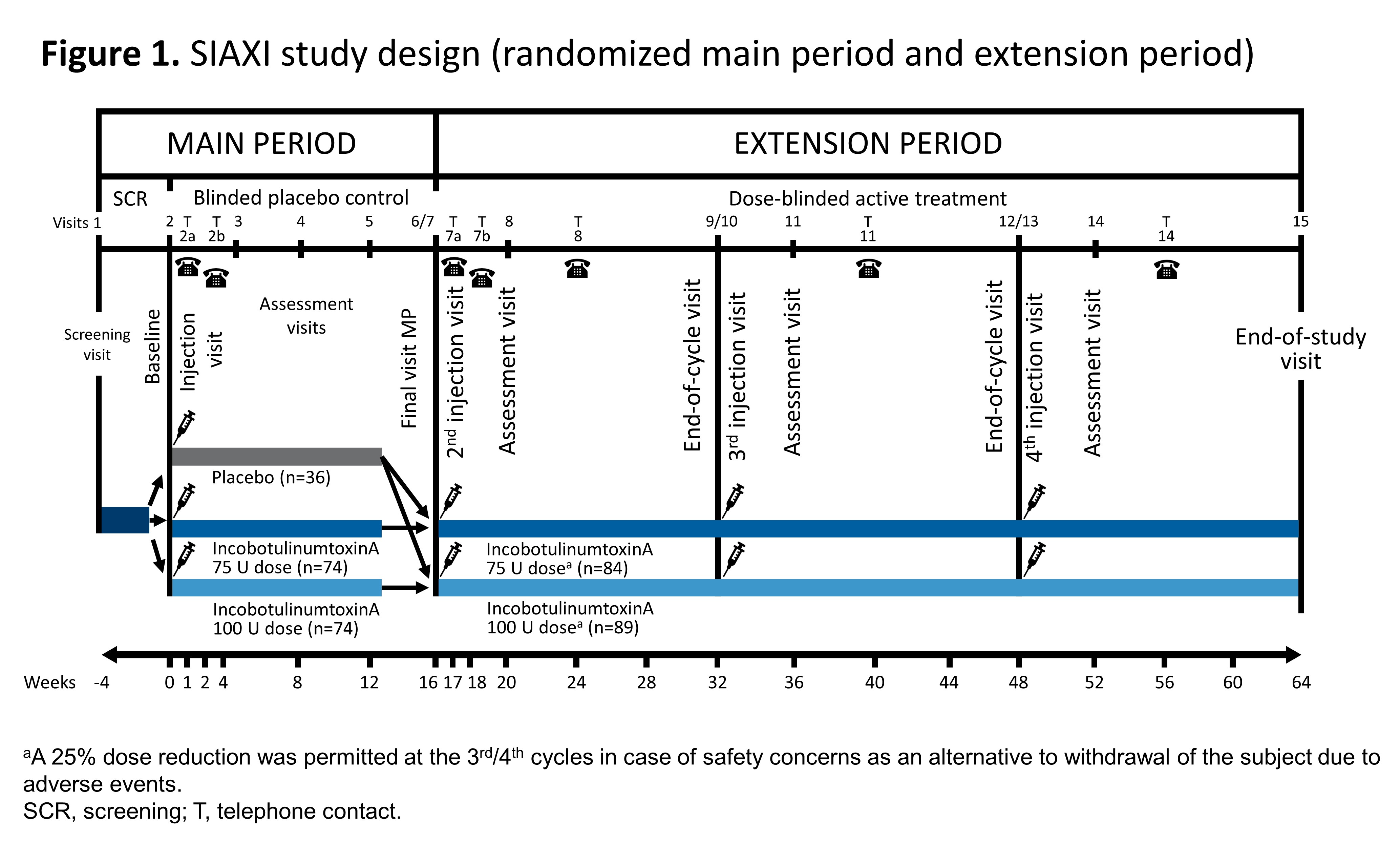
Background: The double-blind, phase lll study SIAXI (NCT02091739) investigated a single injection cycle (IC) of INCO. Eligible patients (pts) completing treatment could receive 3 further INCO ICs in the extension period (EP).
Method: Pts were randomized (2:2:1) to placebo (PL) or INCO 75 or 100U in the main study (16±2 week [wk]) then received either dose for three additional 16±2 wk ICs in the EP (up to 64 wks total) [Figure 1]. Among endpoints assessed were mean unstimulated salivary flow rate (uSFR), modified Radboud Oral Motor Inventory for PD (mROMP) for drooling, speech, and swallowing symptoms and incidence of adverse events (AE). Effect of injection technique (ultrasound [US]- or anatomical landmark [AL]-guided) was also examined.
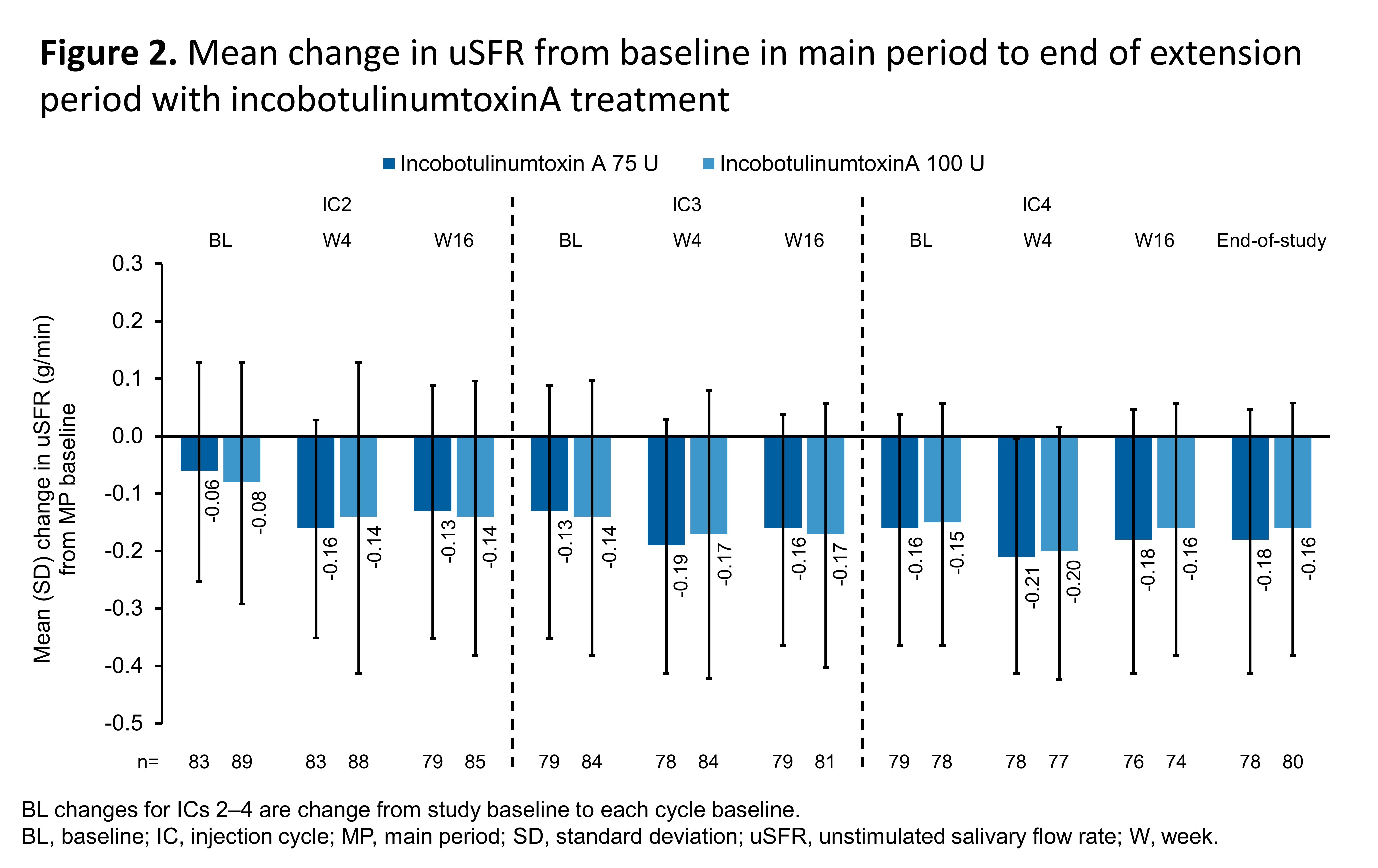
Results: In both INCO 75 and 100U groups (each n=74), mean uSFR reductions occurred at wk4 [p=0.004 vs PL (n=36) for INCO 100U] [Figure 2] and continued consistently in the EP (n=173 total). mROMP drooling scores improved vs PL at wk4 and at all assessments in the EP with INCO 75U, 100U and PL; respective reductions [standard deviation, SD] were greatest at wk8 (–6.29 [6.52], –6.58 [5.90], and –1.26 [4.91]) and wk12 (–6.77 [6.05], –6.40 [5.20], and –1.77 [4.54]). mROMP speech scores improved, with no difference between treatment groups, and swallowing scores remained stable across the study. The most frequent treatment-related AEs were dry mouth and dysphagia. Choice of guidance technique did not impact efficacy (mean uSFR [SD] change from baseline to study end: US: –0.15 [0.20] and –0.17 [0.25]; AL: –0.19 [0.22] and –0.17 [0.20]) or AE rates over all IC (67% and 61% for US- and AL-guided, respectively). No new safety concerns were noted in the EP.
Conclusion: Long-term clinically relevant improvements were seen in adults with chronic sialorrhea associated with PD and other conditions who received INCO treatment. Long-term safety was also confirmed. The choice of injection guidance technique did not influence efficacy or AE rate in our analysis.
To cite this abstract in AMA style:
W. Jost, F. Pagan. IncobotulinumtoxinA for treating chronic sialorrhea due to Parkinson’s disease and other neurological conditions [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/incobotulinumtoxina-for-treating-chronic-sialorrhea-due-to-parkinsons-disease-and-other-neurological-conditions/. Accessed April 28, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/incobotulinumtoxina-for-treating-chronic-sialorrhea-due-to-parkinsons-disease-and-other-neurological-conditions/