Category: Parkinson's Disease: Neurophysiology
Objective: Developing an in vitro α–synuclein (α-s) aggregation and propagation model of human cortical networks for functionality studies.
Background: Studying α–s pathology and its effects on neuronal functionality is critical for understanding the mechanisms behind Parkinson disease (PD) development and progression. Reduction of synaptic proteins, progressive impairments in neuronal excitability, synaptic activity, and network connectivity have been reported [1, 2]. Recently, microfluidics-based chip models enable more advanced experimental setups comparing to conventional in vitro models by controlling the transfer of α–s preformed fibrils (PFFs), isolating synaptically connected neurons and observing the PFF triggered aggregation [3]. More sophisticated platforms combining microfluidic technology with microelectrode arrays (MEA) can provide a disease on chip models as both cellular and functional level dissection is achievable [4].
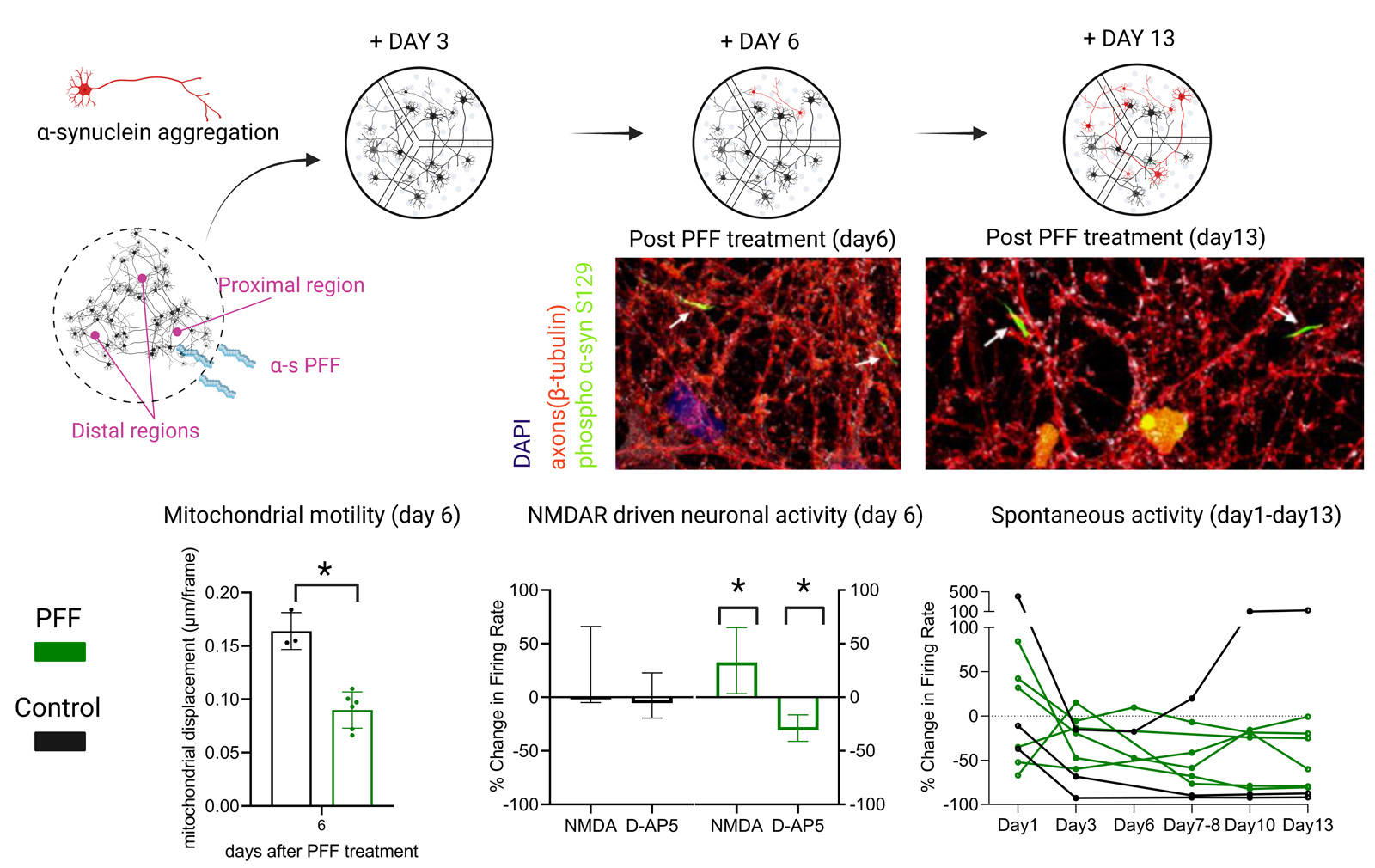
Method: Human induced pluripotent stem cell (hiPSC)-derived cortical neurons were cultured on 3-chambered microfluidics devices with microtunnels between each compartment; thus, established separated networks with axonal connections between each other. S129A mutant PFF was applied to one compartment to induce α–s aggregation in proximal and its spread through axons towards distal networks [figure1]. Embedded MEAs enabled studying functional effects during the spread of pathology. The spread of aggregated/phosphorylated forms of α–s between networks was successfully tracked via phosphorylation at S129 with immunocytochemistry. In parallel to MEA recordings, mitochondrial motility in the axons and calcium activity was assessed together with pre-synaptic protein quantification and cell viability in different phases of aggregation.
Results: Results showed reduction in mitochondrial function and number of presynaptic proteins prior or simultaneous to formation of phosphorylated/aggregated α–s. Diminished neuronal activity was not clear indicator as it has tendency to decrease in all aging networks. However, the response to glutamatergic modulators was distinct between the controls and networks containing phosphorylated/aggregated α–s [figure1].
Conclusion: The model provides temporal data on functional and structural changes during the spread of α–s pathology. Further improvement of the model would provide functional screen for validation of the candidate drugs and would open the way for precision medicines in PD studies.
References: [1] Bendor, J.T., Logan, T.P., Edwards, R.H., 2013. The function of α-synuclein. Neuron. https://doi.org/10.1016/j.neuron.2013.09.004
[2] Peelaerts, W., Bousset, L., Van Der Perren, A., Moskalyuk, A., Pulizzi, R., Giugliano, M., Van Den Haute, C., Melki, R., Baekelandt, V., 2015. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. https://doi.org/10.1038/nature14547
[3] Gribaudo, S., Tixador, P., Bousset, L., Fenyi, A., Lino, P., Melki, R., Peyrin, J.M., Perrier, A.L., 2019. Propagation of α-Synuclein Strains within Human Reconstructed Neuronal Network. Stem Cell Reports. https://doi.org/10.1016/j.stemcr.2018.12.007
[4] Pelkonen, A., Mzezewa, R., Sukki, L., Ryynänen, T., Kreutzer, J., Hyvärinen, T., Vinogradov, A., Aarnos, L., Lekkala, J., Kallio, P., Narkilahti, S., 2020. A modular brain-on-a-chip for modelling epileptic seizures with functionally connected human neuronal networks. Biosensors and Bioelectronics. https://doi.org/10.1016/j.bios.2020.112553
To cite this abstract in AMA style:
F. Kapucu, I. Tujula, O. Kulta, L. Sukki, V. Vuolanto, A. Vinogradov, P. Kallio, S. Narkilahti. In vitro α–synuclein aggregation and propagation model [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/in-vitro-%ce%b1-synuclein-aggregation-and-propagation-model/. Accessed April 27, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/in-vitro-%ce%b1-synuclein-aggregation-and-propagation-model/