Objective: To assess the effect of incobotulinumtoxinA on spasticity-related pain (SRP) using pooled data from three large Phase 3 pediatric studies.
Background: In children/adolescents with cerebral palsy (CP) SRP is common, impacts daily quality of life, and is often neglected.
Method: Ambulant and non-ambulant patients were enrolled (2–17 years of age and with uni-/bilateral CP and Ashworth Scale score ≥2 in clinical patterns for treatment). In TIM (NCT01893411), patients received total body incobotulinumtoxinA doses of ≤16 U/kg (≤400 U) for lower-limb (LL) treatment in two injection cycles (ICs). In TIMO (NCT01905683), new recruits and TIM completers received four ICs with 16–20 U/kg (≤400–500 U) for LL or combined LL and upper-limb (UL) treatment. In XARA (NCT02002884), patients received four ICs with 16–20 U/kg (≤400–500 U) for UL or combined LL/UL treatment. Changes in self-reported (child/adolescent) or observed (parent/caregiver) SRP were assessed using the Questionnaire on Pain caused by Spasticity (QPS) in patients with LL (TIM, TIMO, and XARA) and UL treatment (TIMO and XARA).
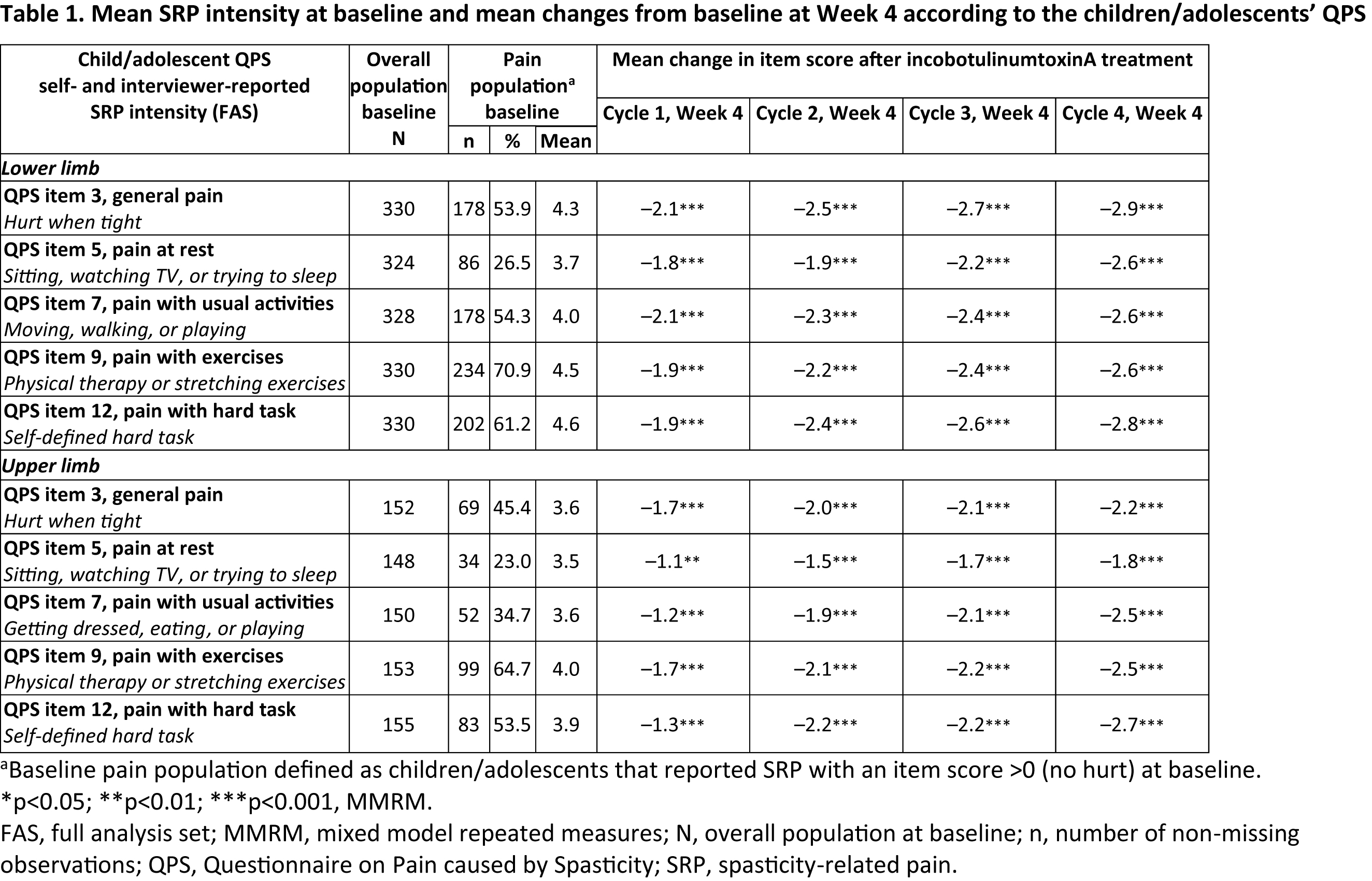
Results: In total, assessments for 849 patients with LL and 454 patients with UL treatment were included. Of these, 340 (40.0%, LL: mean [SD] age 9.3 [3.8] years, 61.2% male, BW 32.6 [14.8] kg) and 160 (35.2%, UL: mean [SD] age 10.3 [3.7] years, 61.9% male, BW 36.8 [16.5] kg) assessed SRP by interviewer- or self-administered QPS. Most (81.9% LL; 69.7% UL) reported pain at baseline for ≥1 activity. Significant reductions from baseline in SRP for all QPS item scores were observed at Week 4 of each IC, after treatment with incobotulinumtoxinA [table1] and a substantial proportion of patients also experienced complete SRP relief (25.0% to 54.8%). Observed SRP frequency was consistent with self-reported SRP and was supported by respective QPS item scores (all p<0.001 for responder rates).
Conclusion: In this large, Phase 3 pooled analysis, repeated incobotulinumtoxinA injections led to sustained pain reduction in children and adolescents with spasticity, with ≤54.8% of patients experiencing complete pain relief in the injected limb during activities.
Presented at TOXINS 2021 (Jan 16–17).
To cite this abstract in AMA style:
F. Heinen, P. Kaňovský, A. Schroeder, H. Chambers, E. Dabrowski, T. Geister, A. Hanschmann, I. Pulte, M. Althaus, M. Banach, D. Gaebler-Spira. Improvement of spasticity-related pain with incobotulinumtoxinA treatment in children/adolescents with cerebral palsy: pooled analysis of Phase 3 studies [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/improvement-of-spasticity-related-pain-with-incobotulinumtoxina-treatment-in-children-adolescents-with-cerebral-palsy-pooled-analysis-of-phase-3-studies/. Accessed April 20, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/improvement-of-spasticity-related-pain-with-incobotulinumtoxina-treatment-in-children-adolescents-with-cerebral-palsy-pooled-analysis-of-phase-3-studies/