Category: Spasticity
Objective: To evaluate impact of incobotulinumtoxinA (incoA) on dressing- and hygiene-related disability (DRE and HYG) in adults with upper-limb spasticity (ULS) based on pooled data from six phase 2 or 3 clinical studies.
Background: Spasticity-associated DRE and HYG are functional impairments that negatively impact patient quality of life. IncoA is an effective treatment for ULS, but until now its role in treating LPOS has not been described.
Method: DRE and HYG data were pooled from six phase 2 or 3 studies (four double-blind, placebo-controlled) of incoA for treating ULS in adults. IncoA was administered over one to four injection cycles, with re-injections given when required. DRE and HYG were assessed at baseline and at 4 weeks post-injection using the Disability Assessment Scale (DAS) domains dressing and hygiene: score 0 (none), 1 (mild), 2 (moderate) and 3 (severe) disability. Response was defined as ≥1-point improvement in DRE/HYG score from baseline. Only data for patients with DRE/HYG at baseline (score ≥1) were analysed. Response rates were compared for incoA versus placebo (PBO) 4 weeks after the first cycle, and evaluated for incoA without PBO control 4 weeks after each of the subsequent three treatment cycles, using Chi-square tests (95% confidence interval [CI]).
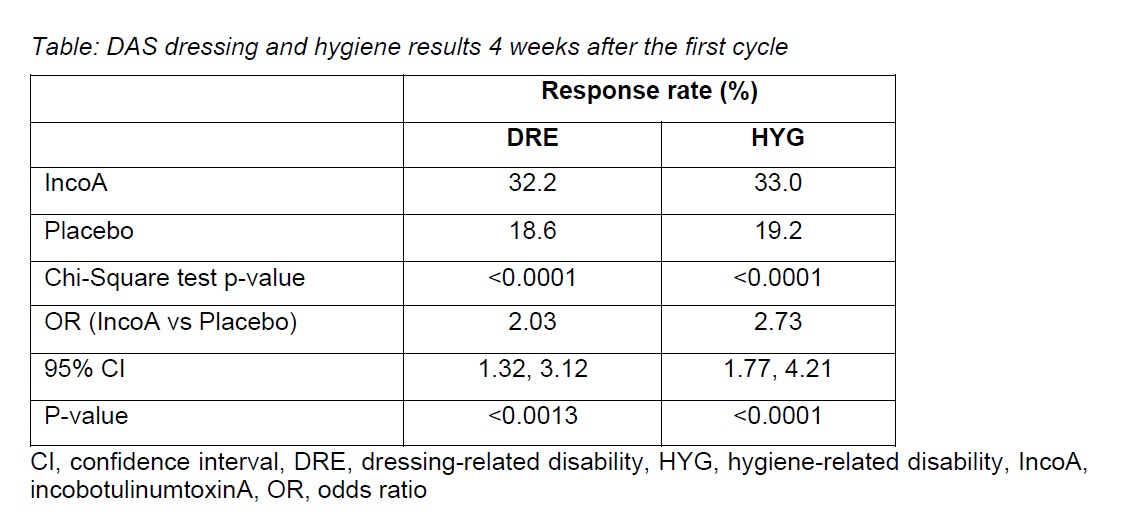
Results: Of the 937 patients at baseline, 907 (96.8%) had DRE (incoA, n=690; PBO, n=217), and 865 (92.3%) had HYG (incoA, n=655; PBO, n=210). IncoA-treated patients were at least twice as likely to achieve a response after the first cycle than PBO-treated patients (Table).
Response rates 4 weeks after the first cycle were significantly higher for incoA vs PBO regardless of DRE baseline severity (p<0.05 for all severity groups) and in patients with moderate or severe HYG at baseline (p<0.05).
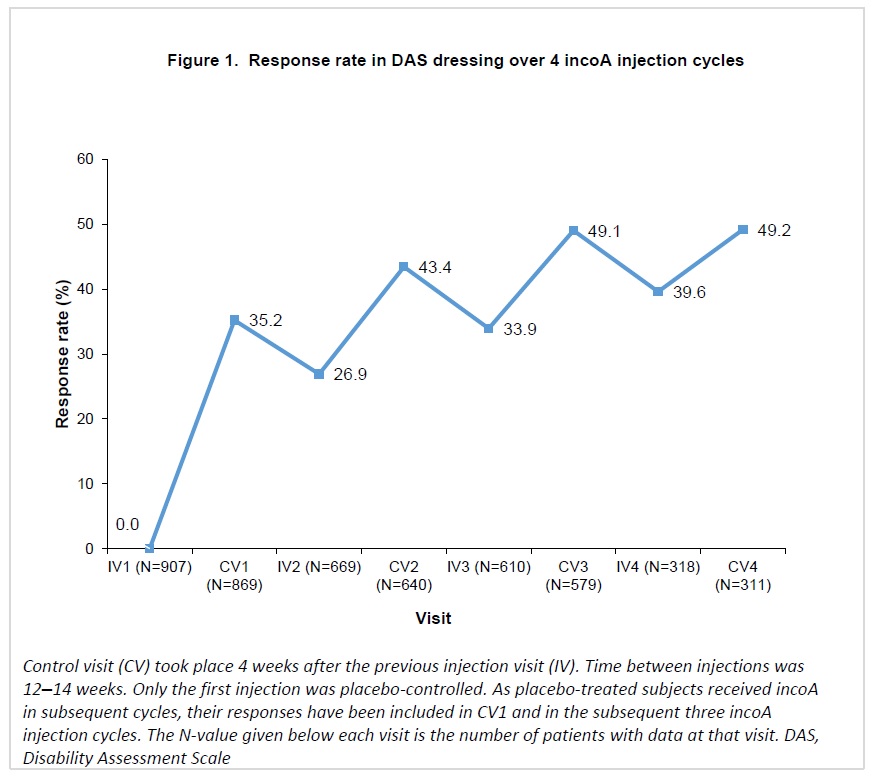
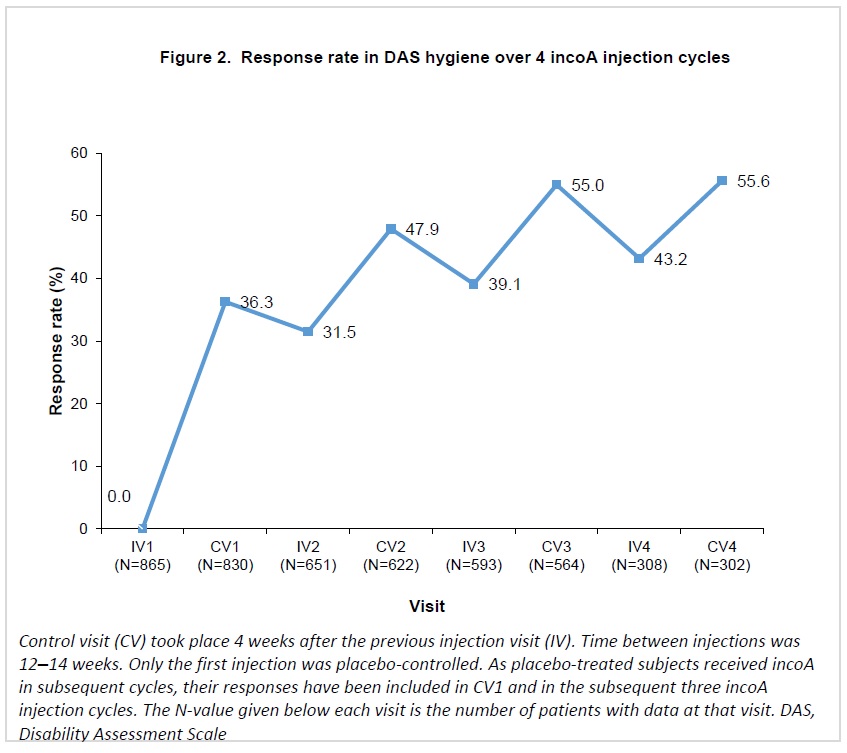
Response rates in DRE increased progressively over repeated incoA treatment cycles, reaching 49.2% 4 weeks after the fourth cycle (Figure 1). For HYG, response rates increased at each cycle, reaching 55.6% 4 weeks after the fourth cycle (Figure 2).
Conclusion: These results support the use of incoA to sustainably improve DRE and HYG, both considered important treatment goals by many patients with ULS.
To cite this abstract in AMA style:
F. Molteni, J. Wissel, K. Fheodoroff, M. Munin, A. Patel, M. Althaus, G. Comes, A. Dekundy, I. Pulte, A. Scheschonka, M. Vacchelli, A. Santamato. Improvement in spasticity-associated dressing- and hygiene-related disability in adults following treatment with incobotulinumtoxinA: a pooled analysis [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/improvement-in-spasticity-associated-dressing-and-hygiene-related-disability-in-adults-following-treatment-with-incobotulinumtoxina-a-pooled-analysis/. Accessed April 20, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/improvement-in-spasticity-associated-dressing-and-hygiene-related-disability-in-adults-following-treatment-with-incobotulinumtoxina-a-pooled-analysis/