Category: Parkinson’s Disease: Clinical Trials
Objective: An exploratory analysis to examine the MDS-UPDRS 1-3 subitems of PD patients who underwent specialized light therapy (SLT) to assess the breadth of impact of SLT on motor and non-motor symptoms.
Background: Several trials of light therapy have been conducted in PD patients using a variety of endpoints. These studies indicate benefit of PD on a variety of motor and non-motor symptoms; however, each study reports only one to a few endpoints. We previously reported the MDS-UPDRS 1-3 combined scores for a SLT PD study [1] and expand by reporting each of the subitems to better evaluate the impact of SLT.
Method: A prospective, randomized, double-blind, multi-center, controlled study was conducted on PD subjects on dopaminergic therapy without significant motor complications. Participants were randomized 1:1 to narrow band blue/green light (active) or low intensity white light (control), thought to not provide clinical benefit for one hour in the evening for 6 months. The previously reported primary endpoint was change from baseline (BL) to 6 months (6M) in the MDS-UPDRS 1-3. Significance of the subitem analysis was considered at p<0.05, trending significance was p<0.10 and no corrections for multiple comparisons were done.
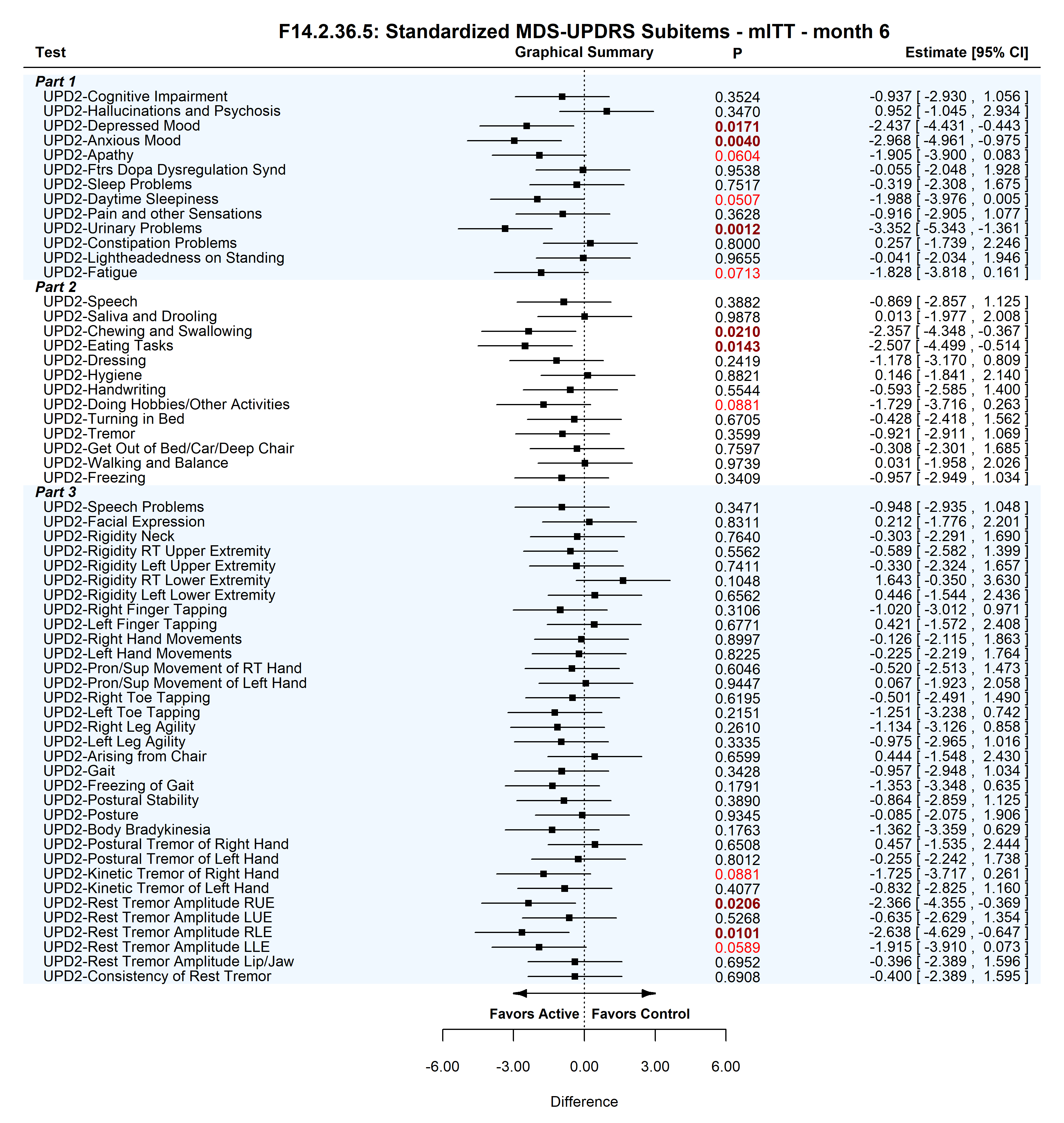
Results: 92 subjects (45 active, 47 control) were enrolled at 3 centers. The primary endpoint MDS-UPDRS 1-3 improved by 17.7(2.8) for the active vs 9.7(3.4) for control. The difference of 8.0(4.4) trended towards significance P=0.074. The subitems for each of the part of the MDS-UPDRS are shown in [figure 1]. MDS-UPDRS 1 has 13 subparts, 3 subitems favored and another 3 trended for SLT. MDS-UPDRS 2 has 13 subparts, 2 favored and another 1 trended for SLT. MDS-UPDRS 3 has 33 subparts, 2 favored and another 2 trended for SLT. In total the MDS-UPDRS 1-3 has 59 subitems, 7 favored and 6 trended in favor of SLT. None of the 59 subitems favored or trended in favor for control.
Conclusion: SLT had a broad impact across a broad range of subitems measured by MDS-UPDRS. Those that significantly favored SLT treatment include depressed mood, anxious mood, urinary problems, chewing and swallowing, eating tasks, rest tremor amplitude RUE, rest tremor amplitude RLE. Daytime sleepiness and fatigue which are typically worsened by dopaminergic treatments, trended in favor of SLT. If confirmed in larger SLT studies this would represent a broad ranging impact to the treatment of motor and non-motor symptoms in PD.
References: [1] Videnovic A, Rutten S, Croft W, Erickson H, Groves J, Havemann C, Herrington T, Hendrix S, Kieburtz K, Van der Werf Y, Van den Heuvel O. Double-blind controlled trial of Spectramax™ light therapy for the treatment of Parkinson’s disease patients on stable dopaminergic therapy, Mov Disord. 2018 Oct;33 Suppl 2:S429
To cite this abstract in AMA style:
B. Wyman, S. Hendrix, S. Hennessey, S. Dickson, J. Groves, W. Croft, D. Adams. Impact of specialized light therapy in Parkinson’s disease on MDS-UPDRS parts 1-3 subscales [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/impact-of-specialized-light-therapy-in-parkinsons-disease-on-mds-updrs-parts-1-3-subscales/. Accessed April 26, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/impact-of-specialized-light-therapy-in-parkinsons-disease-on-mds-updrs-parts-1-3-subscales/