Session Information
Date: Saturday, October 6, 2018
Session Title: Surgical Therapy: Parkinson's Disease
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: Summarize change in quality of life (QoL) by comparing scores from the EuroQuol-five domain questionnaire, five level response version (EQ-5D-5L) summary index obtained at baseline for deep brain stimulation (DBS) naive PD patients implanted with bilateral leads to those scores obtained at 6 &/or 12-month post-DBS, as well as characterize QoL by treatment time and DBS anatomical target (STN vs. GPi).
Background: PD is a progressive disease marked by increased motor complications and disability over time. Understanding the impact of DBS on QOL is an important consideration for patients, health care providers and reimbursement groups. Following publication of the EARLYSTIM data, there is a need to further understand the impact of disease duration (≤ 7.5 years or > 7.5 years), and the associated change in QOL from a real-world population. Impact of QoL based on DBS anatomical target can also be analyzed.
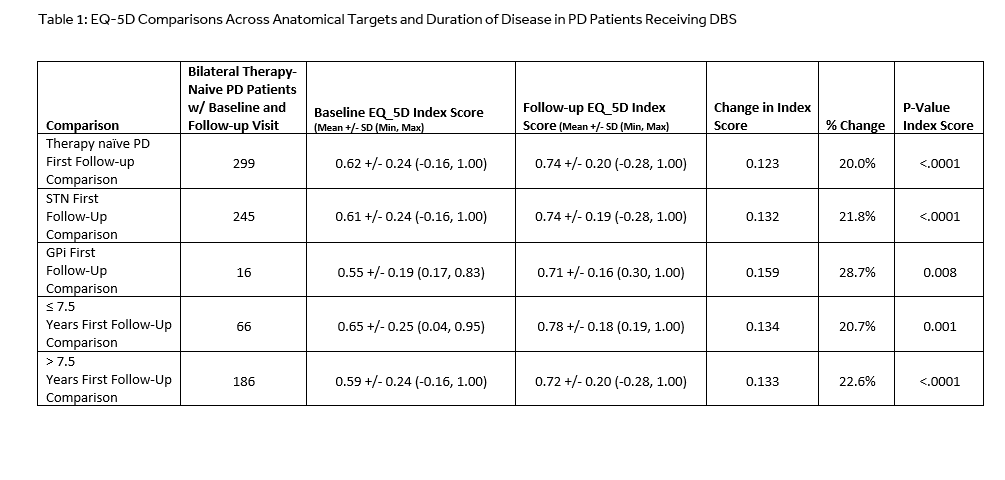
Methods: The Product Surveillance Registry (PSR) is a prospective, long-term, multi-center global registry originated for DBS in 2009. EQ-5D-5L questionnaire, added in 2013, represents data from 544 patients collected at 26 centers who were implanted with a DBS device for PD from 2013-2017. Five domains of the EQ-5D-5L include mobility, self-care, usual activities, pain/discomfort, anxiety/depression. EQ-5D-5L summary index is scored -1.0 (worst) to 1.0 (best) scale.
Results: Subject demographics total N=544, (therapy naïve N=420, replacement device N=123, not reported N=1). Mean age at enrollment was 61.3 ± 9.3 years for therapy-naïve and 65.9 ± 10 years for replacement patients. Of the therapy-naïve patients receiving bilateral lead placement, 388 reported STN and 32 reported GPi as the anatomical target. In addition, 110 patients reported disease duration as ≤ 7.5 years (26.1%) and 277 reported > 7.5 years (65.9%). The remaining 7.9% has unknown time or not reported. EQ-5D-5L summary index scores at first follow-up (either 6 or 12-month) improved 0.12 or 20% (p<. 0001) overall. For the STN group, summary index scores at first follow-up improved 0.13 or 22% (p<. 0001) and for GPi scores improved 0.16 or 29% (p=0.008). The difference in scores between STN and GPi was not statistically significant (p=0.47). Quality of life improvement was seen in patients who received DBS later in their disease (0.13 or 23%, p<.0001), as well as those treated ≤7.5 years from onset (0.13 or 21%, p=0.001). The difference between the two groups was not statistically significant (p=0.38). All four groups had clinically meaningful improvement in QoL scores.
Conclusions: Results from the PSR demonstrated significant improvement in EQ-5D index score in both the STN and GPi patients, as well as both patient treatment groups (≤ 7.5 years or > 7.5 years).
References: 1. Schuepbach WMM, Knudsen JRK, Volkmann J, et al. Neurostimulation for Parkinson’s disease with early motor complication. NEJM 2013;368(7):610-622. 2. McClure NS, Al Sayah F, Zie F, et al. Instrument-defined estimates of the minimally important difference for EQ-5D-5L index scores. International Society for Pharmacoeconomics and Outcomes Research (ISPOR), 2017.
To cite this abstract in AMA style:
M. Schiess, S. Falowski, S. Palfi, J. Azulay, A. Lopez-Rios, L. Defebvre, J. Espinoza, K. Sandberg, B. Van Dorn, T. Weaver, P. Konrad, J. Krauss. Impact of Disease Duration and Anatomical Target on Health Related QoL in Patients Receiving DBS: Results from the Product Surveillance Registry [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/impact-of-disease-duration-and-anatomical-target-on-health-related-qol-in-patients-receiving-dbs-results-from-the-product-surveillance-registry/. Accessed April 26, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/impact-of-disease-duration-and-anatomical-target-on-health-related-qol-in-patients-receiving-dbs-results-from-the-product-surveillance-registry/