Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate the efficacy of home health DBS postoperative management in an effort to reduce travel burden and improve access.
Background: The travel required to receive deep brain stimulation (DBS) programming is a substantial burden on patients, and limits those who can access DBS therapy.f
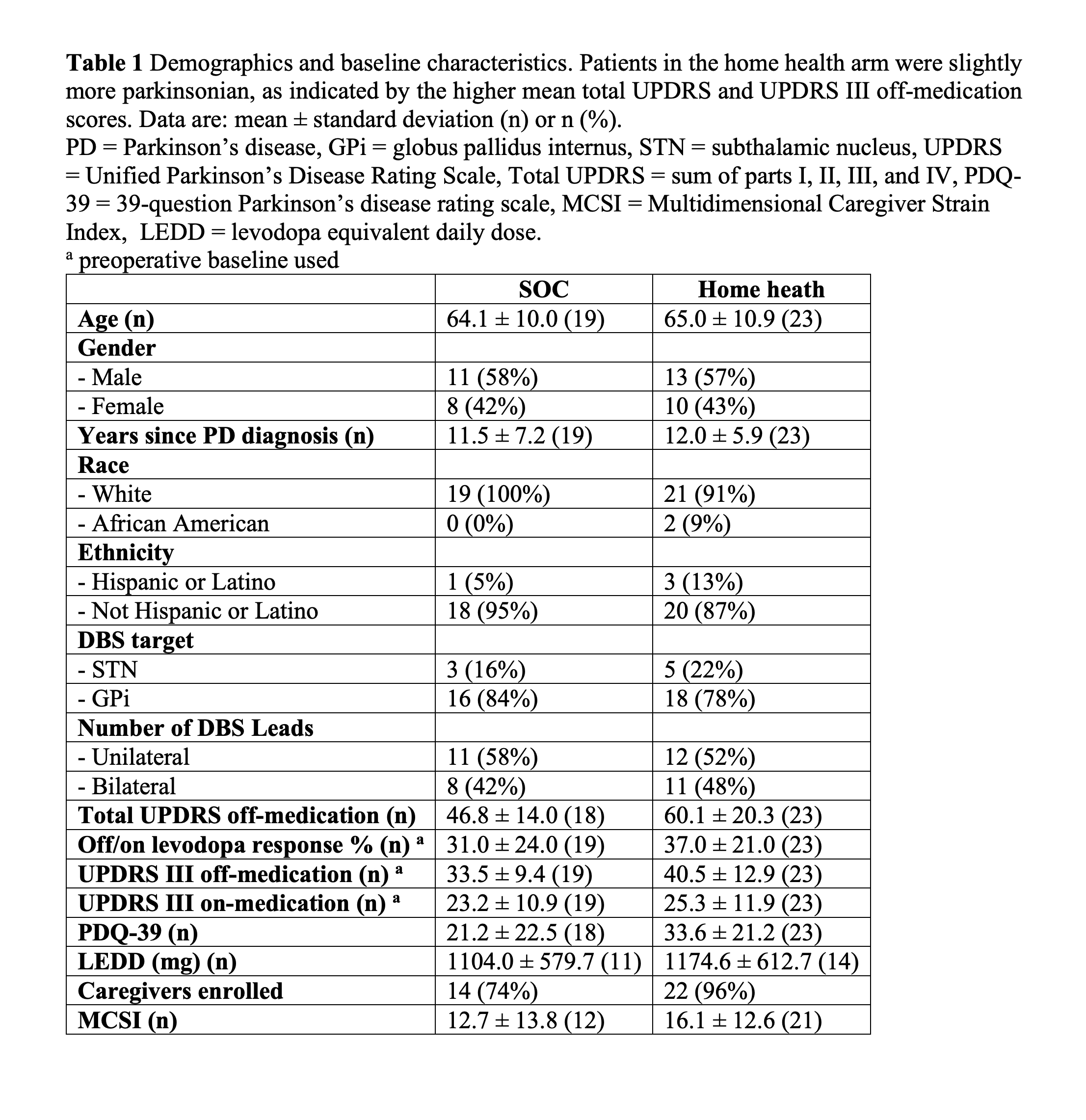
Method: We conducted an open-label, randomized clinical trial at the University of Florida (UF) Health from 2017 to 2020. Consenting participants receiving DBS as part of standard of care treatment for Parkinson’s disease were 1:1 randomized to receive either standard of care (SOC) or home health postoperative DBS management for the first six months after surgery. Primary caregivers, usually the spouse, were also enrolled to assess caregiver strain. The home health postoperative management was conducted by a home health nurse who chose DBS settings with the aid of the iPad based Mobile Application for PD DBS (MAP DBS) system. Prior to the study, the home health nurse had no experience providing DBS care. The primary outcome was the number of times each patient traveled to the movement disorders clinic during the study period. Secondary outcomes included changes from baseline in the Unified Parkinson’s Disease Rating Scale part III (UPDRS III).
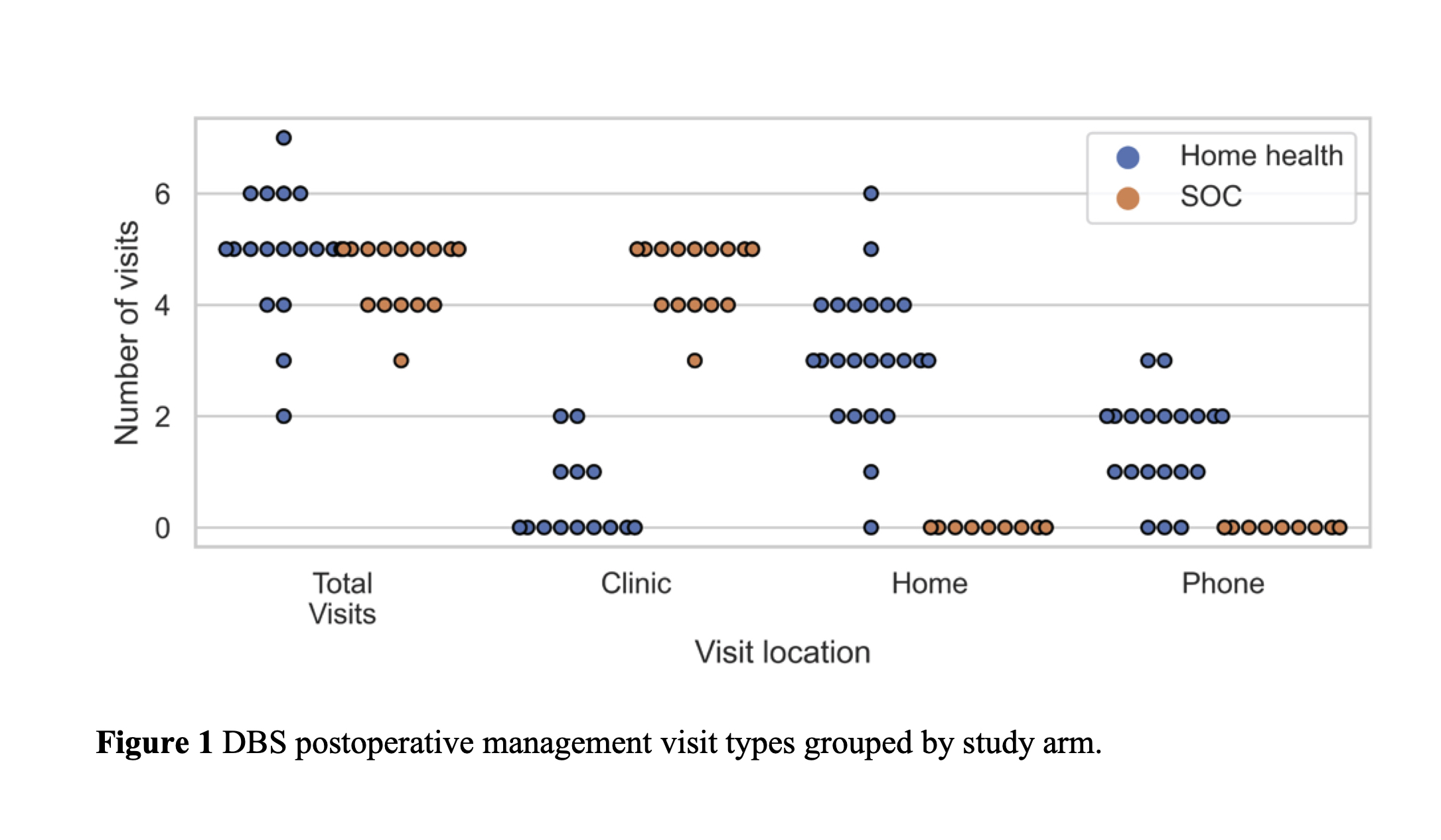
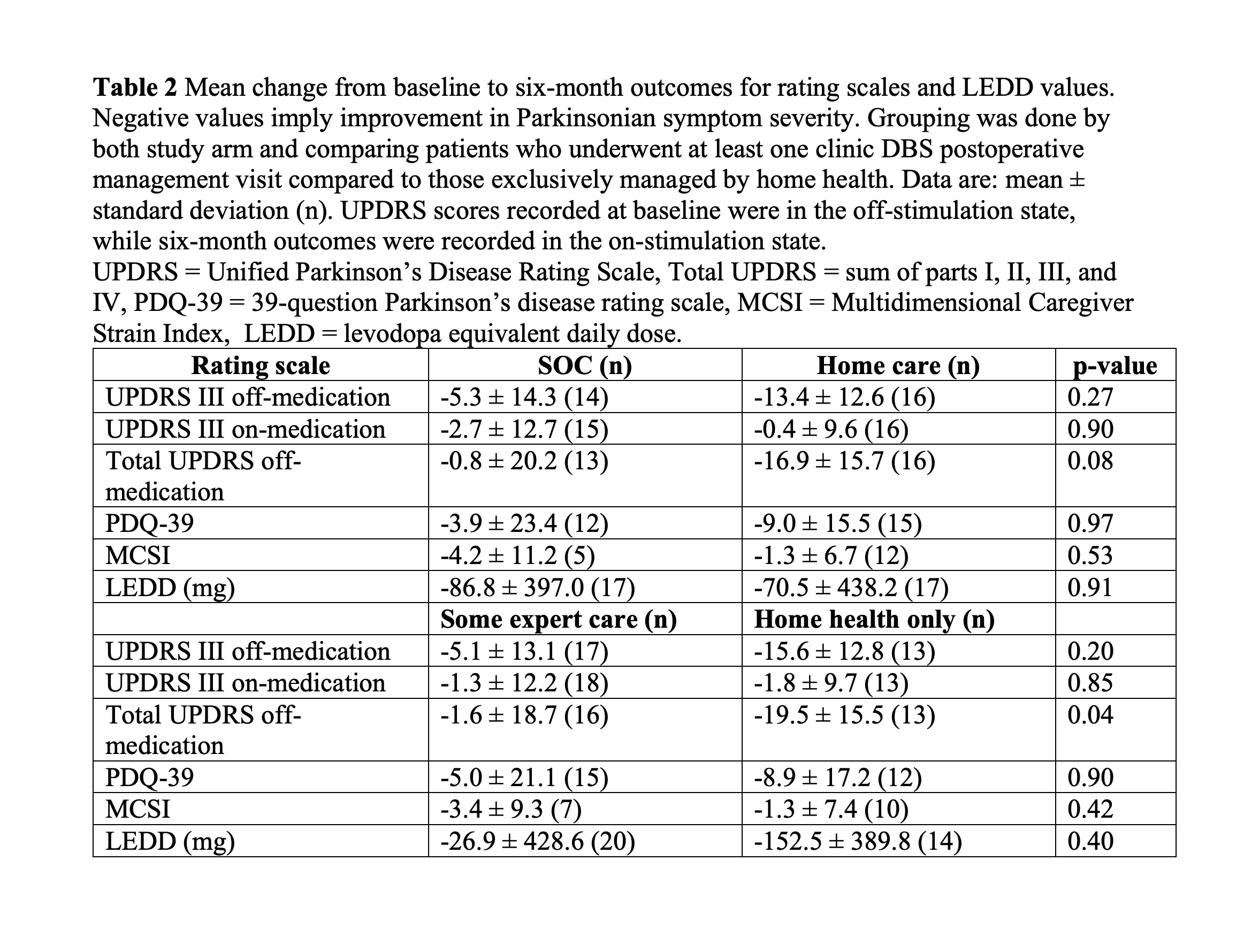
Results: A total of 44 patients were enrolled during the study. Twenty-one patients were randomized to SOC DBS and 23 were randomized to home health postoperative management, which included two in person visits and three phone based visits. We analyzed 19 SOC and 23 home health patients who underwent a minimum of one postoperative management visit. The primary outcome revealed that patients randomized to home health had significantly fewer clinic visits than the SOC arm patients (SOC: 4.8 ± 0.4, home health: 0.4 ± 0.8, p < 0.0001). We found no significant differences between the groups in the secondary outcomes measuring the efficacy of DBS. No adverse events were related to the study procedure or devices.
Conclusion: This study provides evidence supporting the safety and feasibility of postoperative, home health DBS management.
To cite this abstract in AMA style:
G. Duffley, A. Wright, C. Hess, A. Ramirez-Zamora, P. Zeilman, S. Chiu, A. Szabo, B. Lutz, K. Foote, M. Okun, C. Butson. Home Health Management of Parkinson’s Disease Deep Brain Stimulation: A Randomized Clinical Trial [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/home-health-management-of-parkinsons-disease-deep-brain-stimulation-a-randomized-clinical-trial/. Accessed January 17, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/home-health-management-of-parkinsons-disease-deep-brain-stimulation-a-randomized-clinical-trial/