Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate the effects of Helicobacter pylori eradication on PD symptoms.
Background: Globally, H. pylori infection remains a common problem. Cross-sectional studies in Parkinson’s disease (PD) found worse motor outcomes in infected patients, and small trials reported improved motor function after eradication therapy; however, high-quality evidence is scarce.
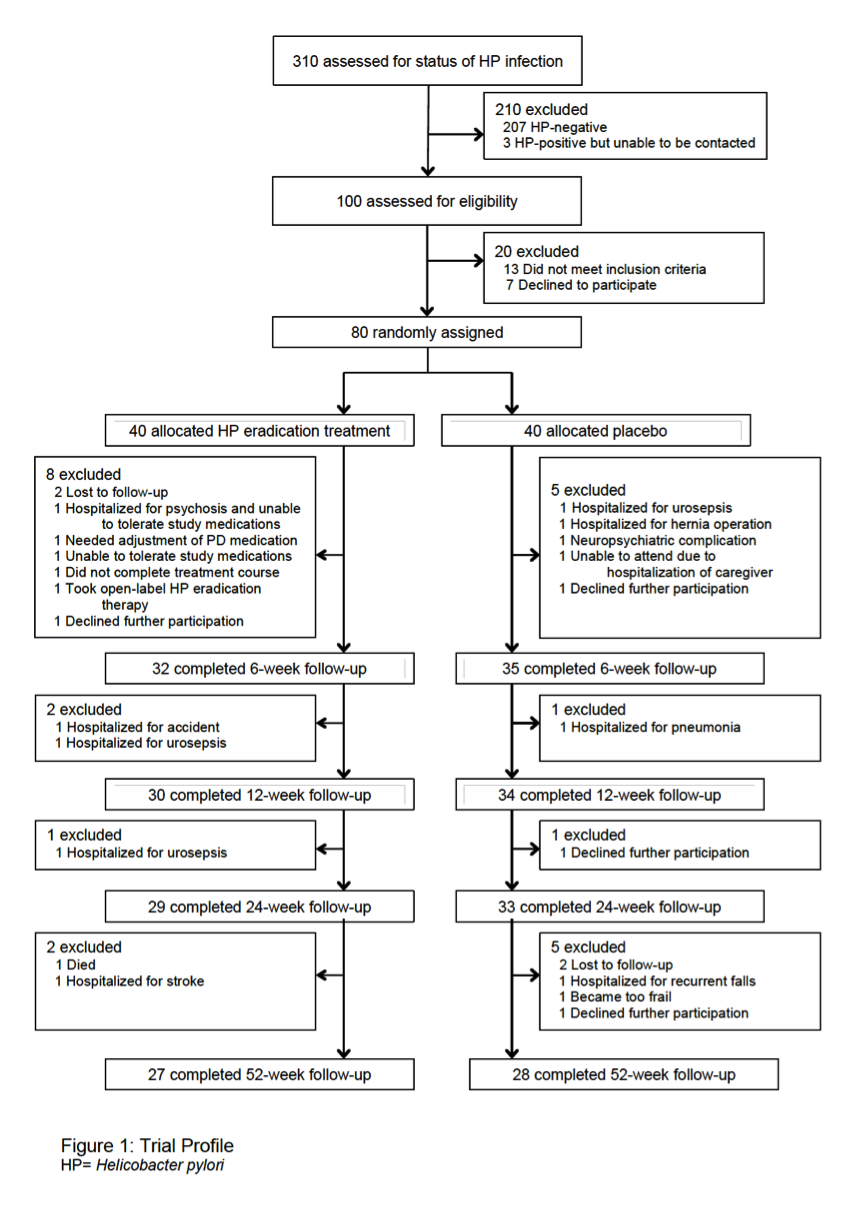
Method: In this parallel group, double-blind, randomized placebo-controlled single-centre trial, PD patients with positive 13C urea breath test and serology for H. pylori were block-randomized (1:1) to receive standard eradication triple therapy or placebo for one week. Capsules were identical in appearance to achieve masking. A variety of pre-specified motor (including timed motor tests and home-based motor function using a wearable sensor), non-motor and quality of life outcome measures were assessed at week 6, 12, 24 and 52. The primary endpoint was baseline-to-week-12 change in ON-medication Movement Disorder Society Unified Parkinson’s disease Rating Scale (MDS-UPDRS) motor scores. Breath testing for concomitant small intestinal bacterial overgrowth (SIBO) was performed at baseline, and was repeated for H. pylori and SIBO at 24 weeks. This study is registered with ClinicalTrials.gov (NCT02108704).
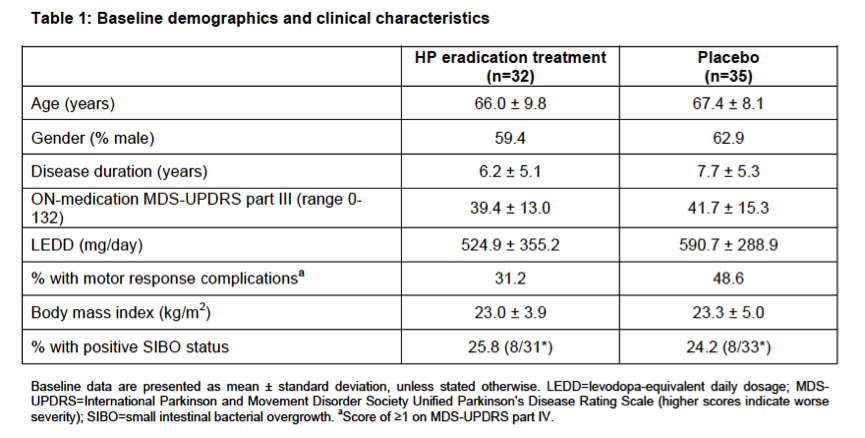
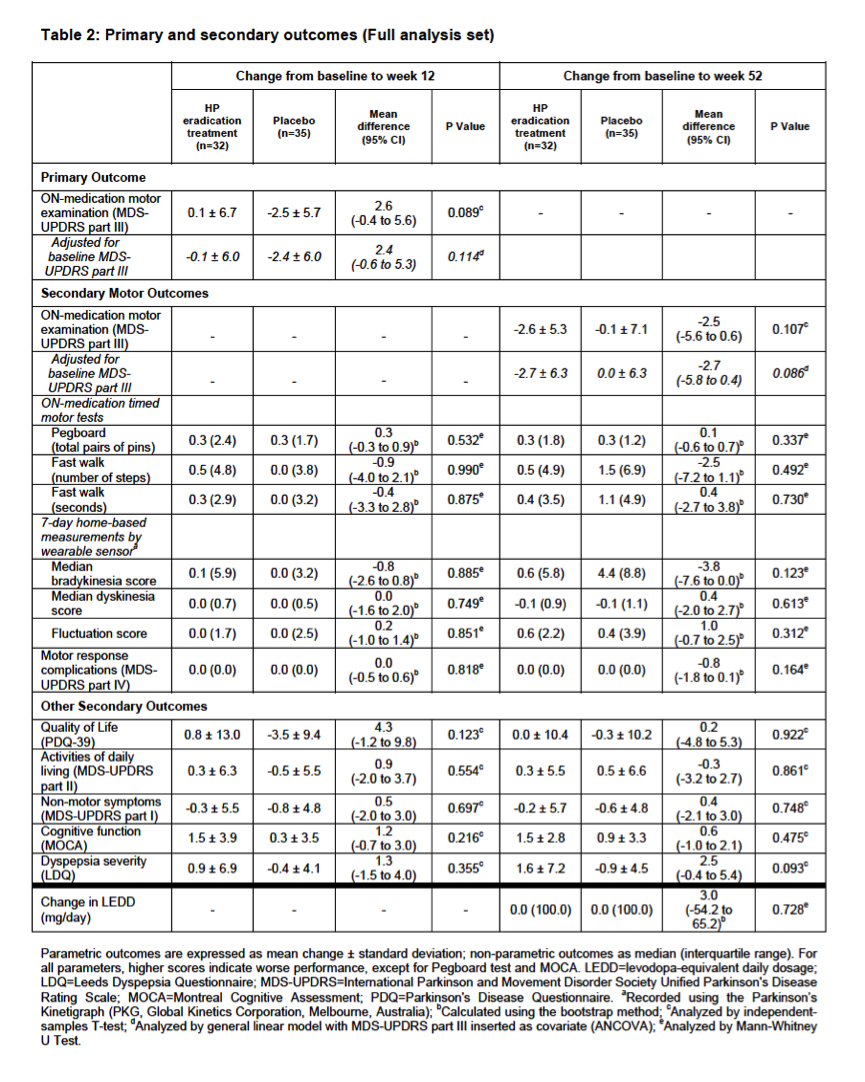
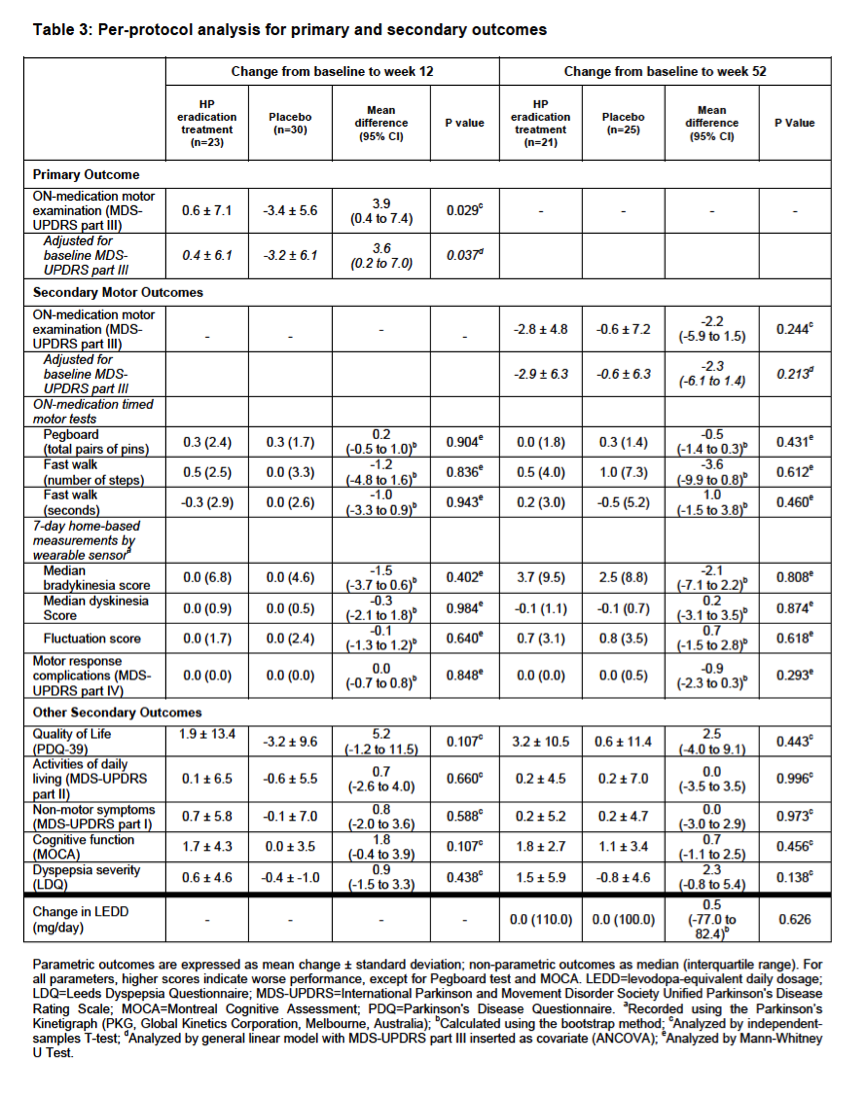
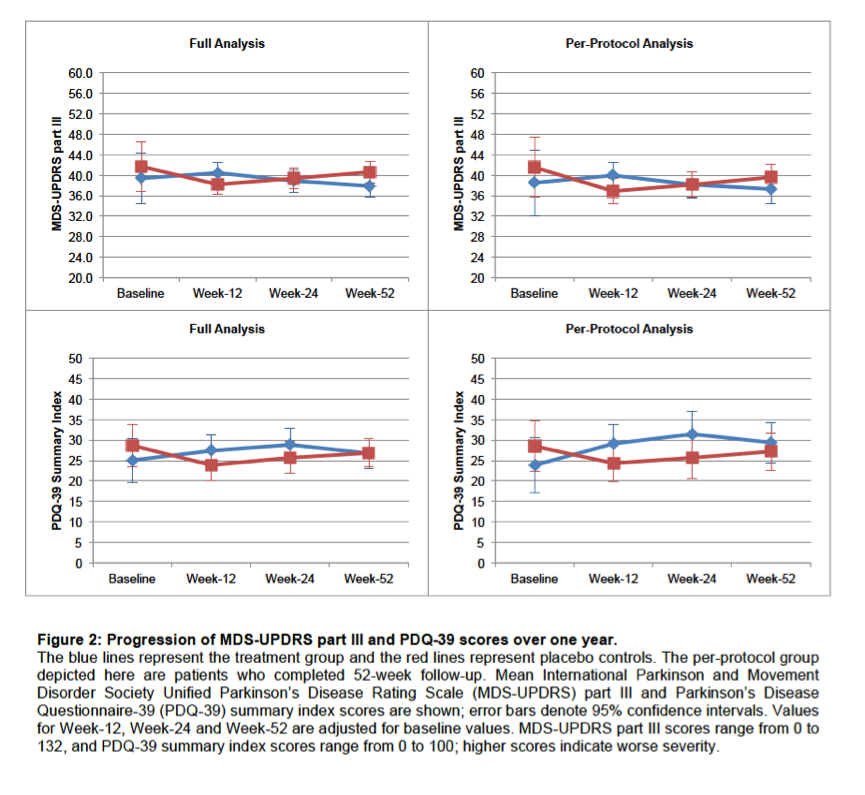
Results: Between December 7, 2013 and May 4, 2016, 310 patients were screened for eligibility and 80 were randomly assigned, of whom 67 were included in the full-analysis set (32 treatment group, 35 placebo). Eradication treatment did not improve MDS-UPDRS motor scores at 12 weeks (mean worsening of 0.1 points [SD 6.7] with treatment vs. improvement of 2.5 points [SD 5.7] with placebo, difference 2.6 points, 95%CI: -0.4 to 5.6, P=0.089). There was no significant improvement in any measures of motor performance, non-motor features, or quality of life at 12- and 52-week timepoints. Both the full-analysis and per-protocol analyses (based on eradication status) supported these conclusions. Presence of SIBO did not influence treatment results and spontaneous changes in SIBO status were observed in the placebo group.
Conclusion: For people with Parkinson’s disease, H. pylori eradication does not improve clinical outcomes, suggesting that there is no justification for routine screening for asymptomatic H. pylori infection, nor for H. pylori eradication with the goal of improving PD symptoms.
References: 1. Tan AH, Mahadeva S, Marras C, et al. Helicobacter pylori infection is associated with worse severity of Parkinson’s disease. Parkinsonism Relat Disord 2015; 21: 221-25. 2. Pierantozzi M, Pietroiusti A, Brusa L, et al. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology 2006; 66: 1824-9. 3. Fasano A, Bove F, Gabrielli M, et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord 2013; 28: 1241-9.
To cite this abstract in AMA style:
A.H Tan, S.Y Lim, S. Mahadeva, M.F Loke, J.S Vadivelu, C. Marras, S.H Fox, A.E Lang. Helicobacter pylori eradication in Parkinson’s disease: A double-blind randomized placebo-controlled study [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/helicobacter-pylori-eradication-in-parkinsons-disease-a-double-blind-randomized-placebo-controlled-study/. Accessed April 18, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/helicobacter-pylori-eradication-in-parkinsons-disease-a-double-blind-randomized-placebo-controlled-study/