Session Information
Date: Monday, June 5, 2017
Session Title: Parkinsonism, MSA, PSP (Secondary and Parkinsonism-Plus)
Session Time: 1:45pm-3:15pm
Location: Exhibit Hall C
Objective: To investigate the gut microbiome and metabolome in MSA
Background: Experimental models of prion-like cell-to-cell transfer of alpha-synuclein(AS) and its ability to spread from the enteric nervous system(ENS) to the brain, have reignited Braak’s proposal that environmental insults acting on the ENS could trigger the misfolding of AS, subsequently propagating into the central nervous system. This gut-brain-axis in synucleinopathies is further supported by mounting evidence for the role of gut microbiota in regulating diseases via intense bidirectional interplay of neuronal, immunological and hormonal signaling. There is new preclinical evidence that gut microbes promote microglial activation and AS-mediated motor deficits and pathology.1 Three studies have reported alterations of gut microbiome in PD,2 but none has been published in MSA to date.
Methods: We recruited 17 MSA patients and 17 age-matched spouses/siblings(living in the same community, to control for confounding factors such as lifestyle, diet and housing condition). Demographic data and detailed medical history including medications, diet and constipation severity were collected. Stool DNA was extracted and amplicon sequencing targeting the V3-V4 region of the microbial 16SrRNA gene was performed on Illumina Miseq platform. Stool metabolomics were analysed using nuclear magnetic resonance spectroscopy.
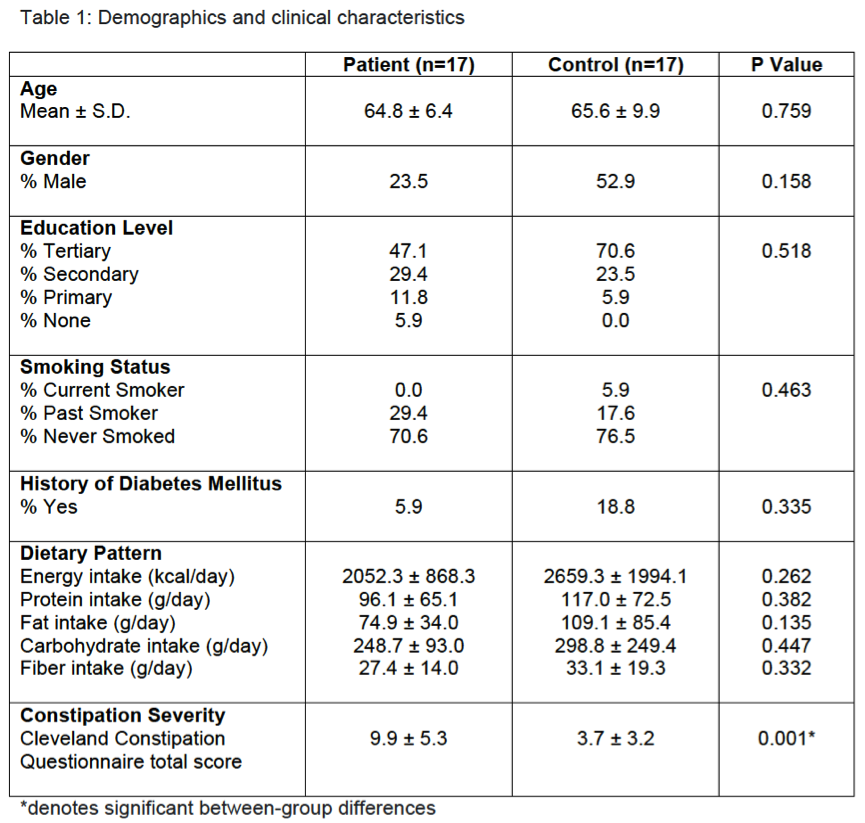
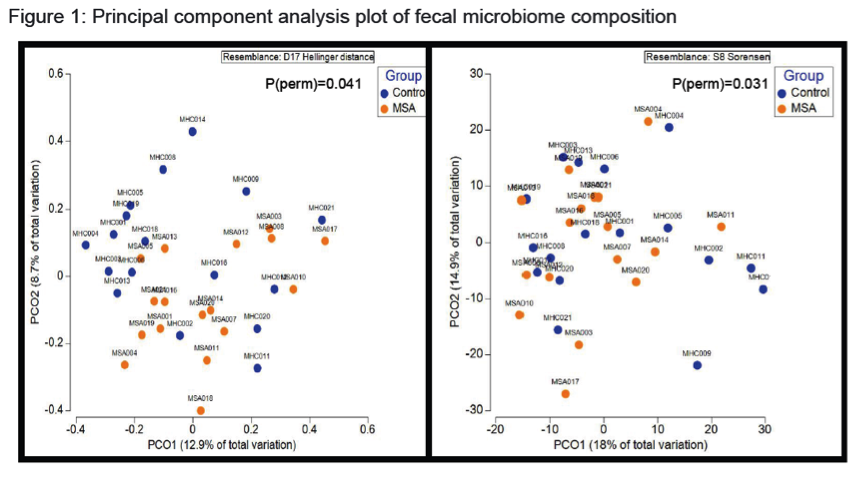
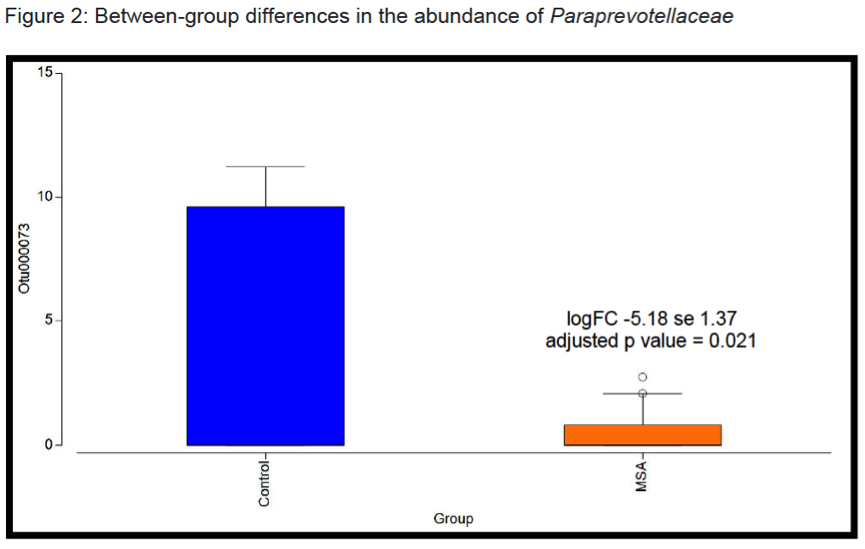
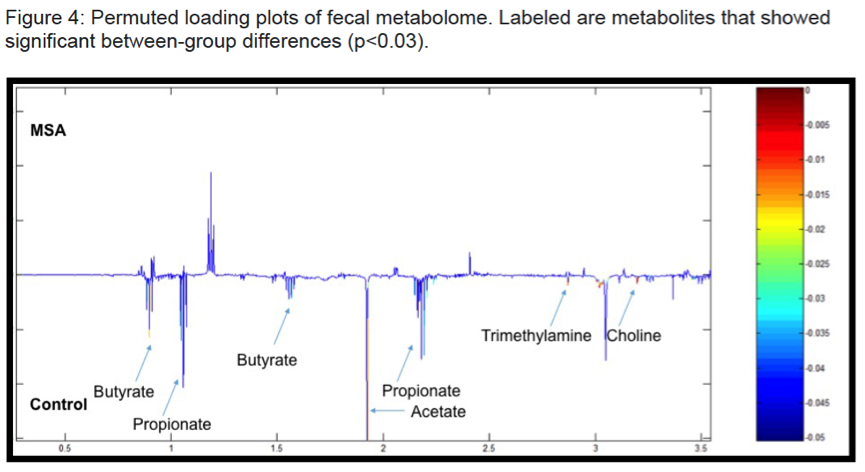
Results: There were no significant group differences in age, gender, education level, dietary pattern, smoking and diabetic status. MSA patients had worse constipation severity score(Table 1). Four patients were treatment-naïve and mean MSA duration was 3.7±2.1years. Fecal microbiota composition was significantly different between patients and controls(Fig 1). MSA patients had a significant 5-fold reduction in the abundance of paraprevotella (p=0.02)(Fig 2). There was no correlation between the abundance of paraprevotella with constipation severity(r=0.185,p=0.295). We found a clear separation in fecal metabolomics between the two groups(Fig 3), providing further insights into differences in bacterial functions. MSA patients had significantly lower levels of fecal butyrate, propionate, trimethylamine and choline(Fig 4).
Conclusions: Preliminary results suggest an alteration of gut microbiota in MSA patients. The finding of reduced abundance of paraprevotella mirrors a previous study in PD.2 The potential role of the gut in the etiopathogenesis of synucleinopathies warrants further study.
References:
- Sampson TR, Debelius JW, Thron T et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s Disease. Cell 2016; 167:1469-1480.
- Scheperjans F. Gut microbiota, 1013 new pieces in the Parkinson’s disease puzzle. Curr Opin Neurol 2016; 29:773-780.
To cite this abstract in AMA style:
A.H. Tan, C.W. Chong, S.-L. Song, C.S.-J. Teh, I.K.-S. Yap, M.F. Loke, Y.Q. Tan, H.S. Yong, S. Mahadeva, A.E. Lang, S.-Y. Lim. Gut Microbiota in Multiple System Atrophy [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/gut-microbiota-in-multiple-system-atrophy/. Accessed February 27, 2026.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/gut-microbiota-in-multiple-system-atrophy/