Session Information
Date: Tuesday, June 21, 2016
Session Title: Therapy in movement disorders: Gene and cell-based therapies
Session Time: 12:30pm-2:00pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: The objectives of this meta-analysis are: 1) to investigate the efficacy of AAV2-neurturin gene delivery for patients with Parkinson’s disease; 2) to compare AAV2-neurturin delivery in putamen only versus in putamen plus Substantia Nigra; and 3) to compare efficacy of gene delivery before 18 months and after 18 months of the injection.
Background: There is unmet need for developing better treatment strategies for Parkinson’s disease (PD). Gene delivery of neurturin, a natural analogue for glial derived neurotrophic factor, showed promising results in animal models and early open label trials. However, its efficacy has not been confirmed yet.
Methods: We searched PubMed for clinical trials (single arm and controlled trials) assessing the efficacy of AAV2-neurturin gene delivery in patients with PD. Data were extracted from eligible studies and were analyzed using RevMan and open meta[analyst] statistical packages. We conducted meta-regression to investigate whether the improvement in unified Parkinson’s disease rating scale (UPDRS III motor score) was associated with the target of gene delivery or follow up duration.
Results: Four studies (n=123 patients) were pooled in the final analysis. Compared with sham surgery, neurturin gene delivery was not significantly superior before 18 months but after 18-24 months (UPDRS III: MD -0.39, 95% CI [-4.15 to 3.36] vs. MD -4.49, 95% CI [-8.63 to -0.35], respectively; figure 1). Intraputamenal gene delivery was significantly better than gene delivery in putamen plus substantia nigra (UPDRS III: MD -12.76, 95% CI [-15.61 to -9.92] vs. MD -6.72, 95% CI [-10.38 to -3.06]; P=0.01; figure 2). Figure 1: Forest plot shows MD of change in UPDRS III from baseline to endpoints (neurturin vs. sham) with subgroup analysis according to follow up time. 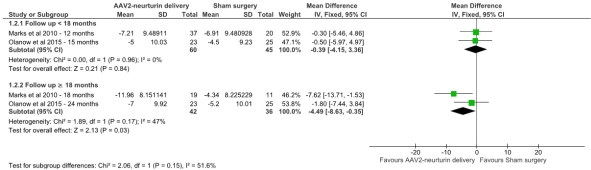 Figure 2: Forest plot shows MD of change in UPDRS III from baseline to endpoint (Putamen only vs. Putamen and Substantia Nigra).
Figure 2: Forest plot shows MD of change in UPDRS III from baseline to endpoint (Putamen only vs. Putamen and Substantia Nigra). 
| STUDY ID | Study designs | Target | End point* | Sample size | UPDRS III | HDAM** | PDQ-39*** |
|---|---|---|---|---|---|---|---|
| Marks 2010 | Randomized controlled trial | Putamen only | 18 | 37/20 | -11.96 (8.15) | -1.92 (3.18) | NR |
| Olanow 2015 | Randomized controlled trial | Putamen and Substantia Nigra | 24 | 23/25 | -7 (9.92) | -1.9 (2.55) | -1.1 (10.39) |
| Raymond 2013 | Open label single arm trial | Putamen and Substantia Nigra | 24 | 6 | -5.5 (10.68) | -2.3 (1.83) | 0 (6.63) |
| Marks 2008 | Open label single arm trial | Putamen only | 12 | 12 | -14 (8) | NR | NR |
Conclusions: Current evidence is insufficient to confirm the efficacy of AAV2-neurturin gene delivery. Our results suggest that the efficacy of AAV2-neurturin gene delivery in the putamen only should be evaluated by further randomized controlled trials with follow up duration beyond 18 months.
To cite this abstract in AMA style:
A.G. Almraezy, H. Ahmed, M. Elnenny, A. Negida. Gene delivery of AAV2-neurturin for patients with Parkinson’s disease: A meta-analysis [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/gene-delivery-of-aav2-neurturin-for-patients-with-parkinsons-disease-a-meta-analysis/. Accessed April 26, 2025.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/gene-delivery-of-aav2-neurturin-for-patients-with-parkinsons-disease-a-meta-analysis/
