Category: Parkinson's Disease: Neuroimaging
Objective: Investigate functional connectivity (FC) fingerprints of Minor Hallucinations (MH) in Parkinson’s Disease (PD).
Background: MHs occur at the early stages of Parkinson’s disease in ~40% of patients, and have been showed to be predictive of later cognitive decline and psychosis1,2. A better understanding of the brain mechanisms of MHs is therefore important to predict these negative clinical outcomes. Past work focusing on group-differences has shown that alterations in FC in fronto-temporal and medial parietal regions are associated with MH in PD3,4, yet it is known that FC-patterns are individual-specific also during neurodegeneration (also known as FC-fingerprints5,6). In this work, we switch the focus from group differences to individual variability and analyse FC-fingerprints in patients with PD-MH and PD-nH, for a better characterization of the neural basis of MH, after taking into account individual variability.
Method: 33 patients with PD were included (PD-MH, 18; PD-nH, N=14). MH were stable during the 3 months preceding the start of the study, and included presence hallucinations, passage hallucinations, visual illusions and/or pareidolias. There were no group differences for demographic and clinical measures (cf. Table 1). We estimated individual FC matrices using Pearson’s correlation between the averaged BOLD signals of 278 cortical and subcortical nodes7, and FC-fingerprints using metrics introduced and estimated in healthy subjects and neurological patients in previous work6,8.
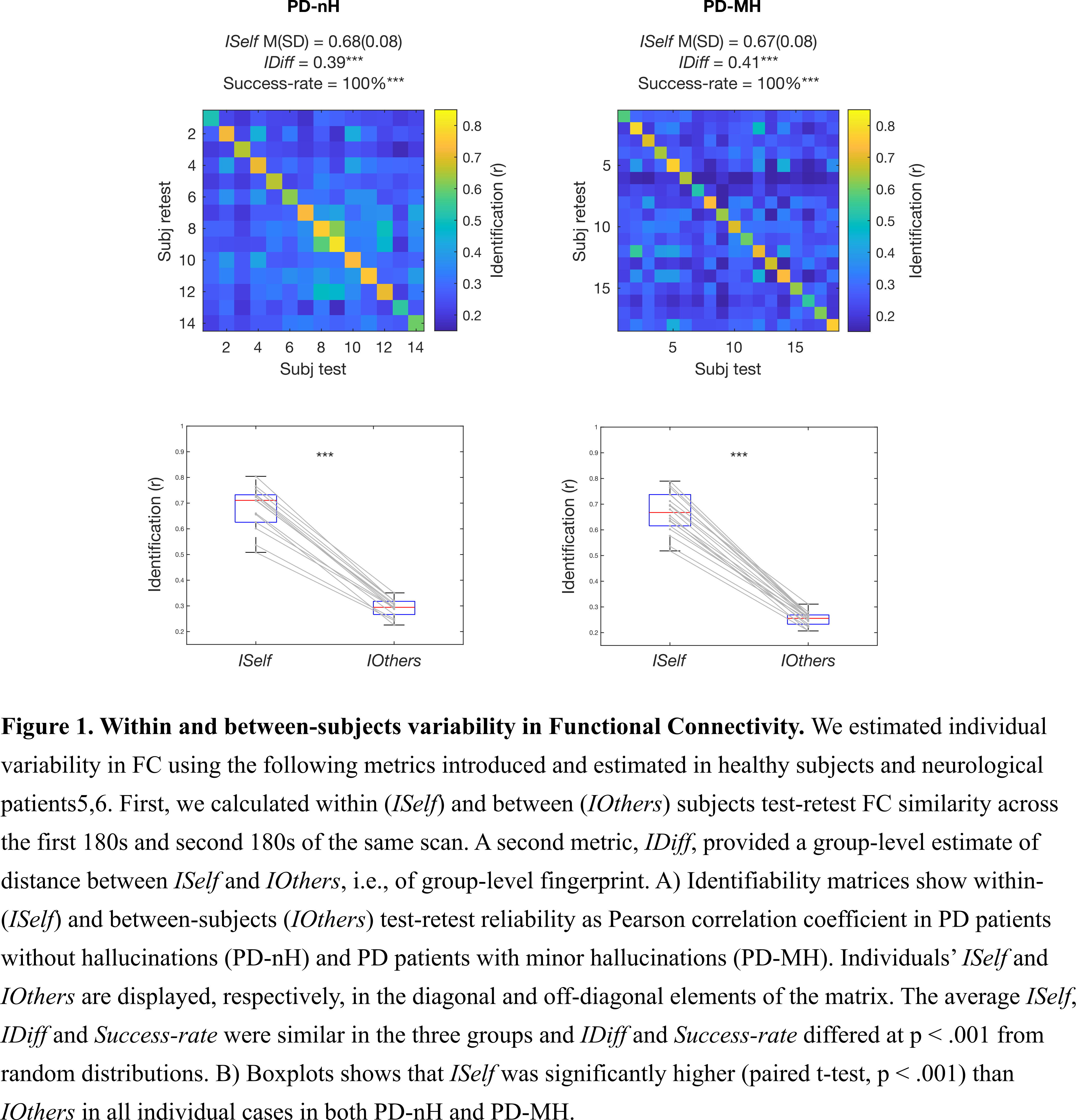
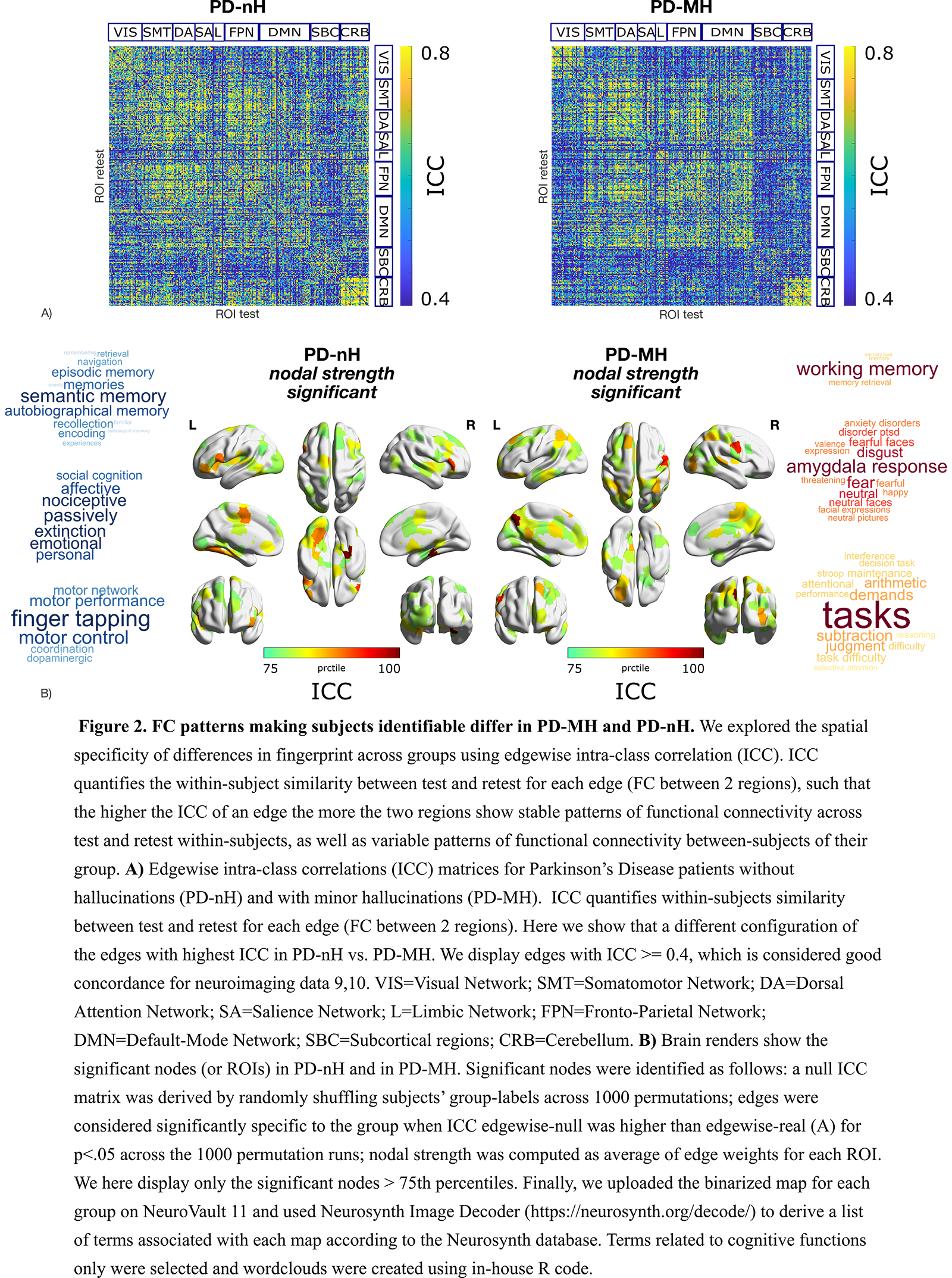
Results: First, data showed that individual FC profiles were highly distinguishable in both PD-MH and PD-nH, i.e., among patients with the same clinical phenotype (Fig.1). Second, we found that FC patterns that identified patients differed between PD-MH and PD-nH (Fig.2A). Regions with higher FC-fingerprint in PD-MH were regions implicated in frontal-executive functioning, short-term memory and face perception while PD-nH subjects showed greater variability in regions implicated in motor and affective processing (Fig.2B).
Conclusion: In this study we show that FC remains individual-specific also in the presence of PD-nH and PD-MH and that different FC-fingerprint distinguish PD-MH from PD-nH. These findings help characterizing the neural basis of early hallucinations in PD (i.e., MH) and may pave the way for a personalised understanding of the altered brain mechanisms in hallucinations as well as for their early detection.
References: 1. Bernasconi, F. et al. Theta oscillations and minor hallucinations in Parkinson’s disease reveal decrease in frontal lobe functions and later cognitive decline. 2022.11.24.517668 Preprint at https://doi.org/10.1101/2022.11.24.517668 (2022).
2. Lenka, A., Pagonabarraga, J., Pal, P. K., Bejr-Kasem, H. & Kulisvesky, J. Minor hallucinations in Parkinson disease: A subtle symptom with major clinical implications. Neurology 93, 259–266 (2019).
3. Bejr-kasem, H. et al. Disruption of the default mode network and its intrinsic functional connectivity underlies minor hallucinations in Parkinson’s disease. Movement Disorders 34, 78–86 (2019).
4. Bernasconi, F. et al. Robot-induced hallucinations in Parkinson’s disease depend on altered sensorimotor processing in fronto-temporal network. Sci. Transl. Med. 13, eabc8362 (2021).
5. Finn, E. S. et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 18, 1664–1671 (2015).
6. Stampacchia, S. et al. Fingerprinting of brain disease: Connectome identifiability in cognitive decline and neurodegeneration. 2022.02.04.479112 Preprint at https://doi.org/10.1101/2022.02.04.479112 (2022).
7. Shen, X., Tokoglu, F., Papademetris, X. & Constable, R. T. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage 82, 403–415 (2013).
8. Amico, E. & Goñi, J. The quest for identifiability in human functional connectomes. Sci Rep 8, 8254 (2018).
9. McGraw, K. O. & Wong, S. P. Forming Inferences About Some Intraclass Correlation Coefficients. Psychological Methods 17 (1996) doi:1082-989X/96/S3.00.
10. Shrout, P. E. & Fleiss, J. L. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychological Bulletin 86, 420–428 (1979).
11. Gorgolewski, K. J. et al. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics 9, (2015).
To cite this abstract in AMA style:
S. Stampacchia, F. Bernasconi, J. Potheegadoo, J. Pagonabarraga, J. Kulisevsky, D. van Deville, E. Amico, O. Blanke. Functional connectivity fingerprints in Parkinson’s disease patients with hallucinations [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/functional-connectivity-fingerprints-in-parkinsons-disease-patients-with-hallucinations/. Accessed December 22, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/functional-connectivity-fingerprints-in-parkinsons-disease-patients-with-hallucinations/