Session Information
Date: Saturday, October 6, 2018
Session Title: Clinical Trials and Therapy in Movement Disorders
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To evaluate the feasibility and potential efficacy of a software-controlled, solid-state caloric vestibular stimulation (CVS)-mediated brainstem modulation device for the treatment of non-motor and motor symptoms in Parkinson’s disease (PD).
Background: CVS introduces thermal current to the external ear canal which modulates the firing rate of the vestibular nerves, and in turn elicits compensatory responses across cortical and sub-cortical structures. Although conventionally used to diagnose brainstem dysfunction, we recently demonstrated in a single case that 8 weeks of daily CVS therapy can reduce motor and non-motor PD symptoms (1). CVS was administered via a using a solid-state ThermoNeuroModulation (TNM) device, developed by Scion NeuroStim, LLC, that unlike conventional air and water irrigators can be self-administered at home with few side effects and with a high degree of dose control (2). The aim of the present study was to replicate these compelling results in a larger PD group.
Methods: Eligible and consenting participants were randomly allocated 1:1 to a placebo or active arm. After a 4 week baseline period, they self-administered 18 minutes of active or placebo treatment twice daily in the home for 8 weeks. Outcome measures were administered every 4 weeks from the start of baseline to 4 weeks follow-up and also 20 weeks after the cessation of therapy. These outcome measures included the MDS-UPDRS, Non-Motor Symptoms Scale, Montreal Cognitive Assessment and the PDQ-39 among others. All participants were treated with stable levels of dopamine replacement therapy throughout study.
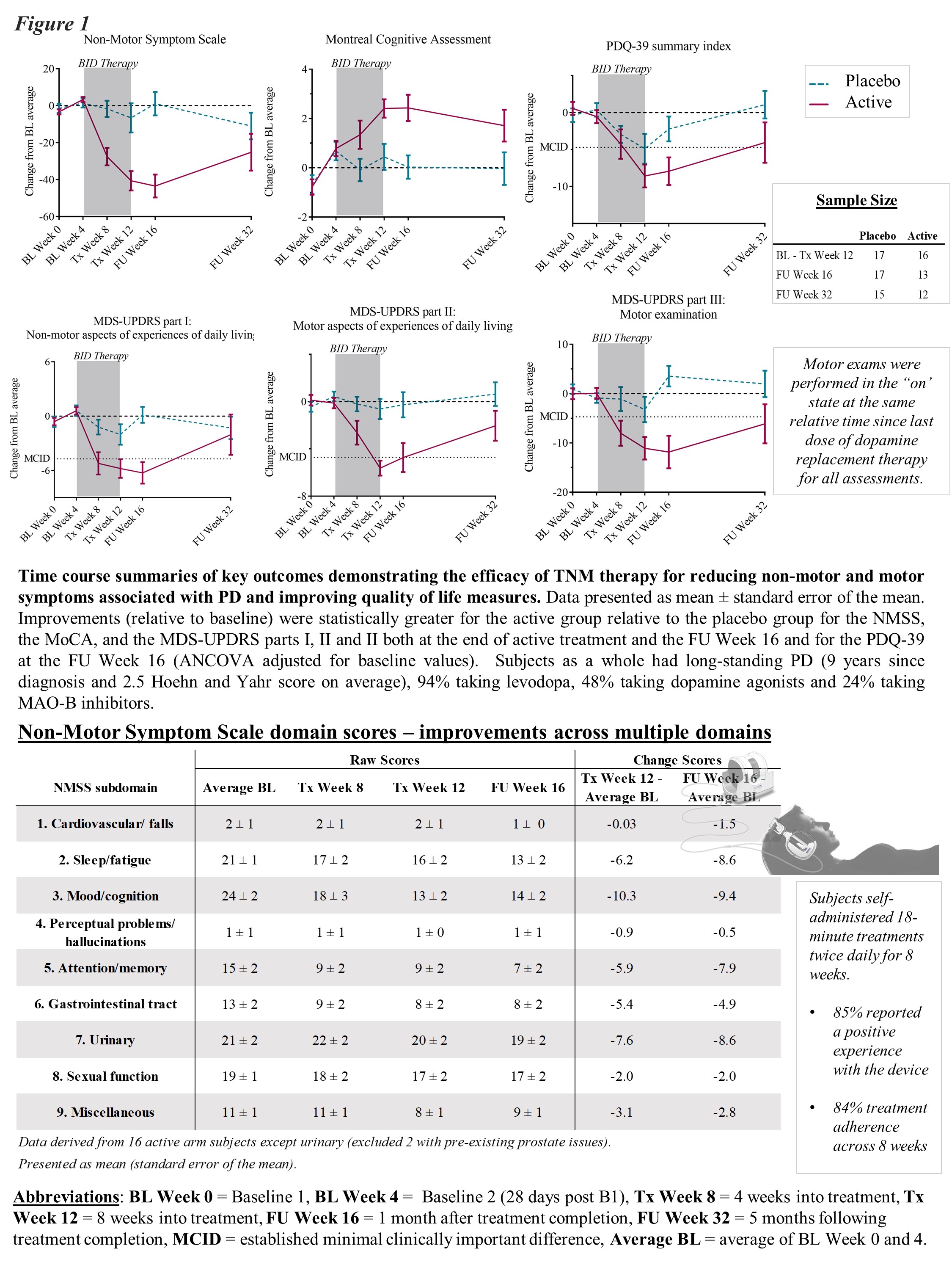
Results: In the per protocol group, active-arm subjects (n = 16) exhibited significantly greater improvements in both motor and non-motor symptoms and activities of daily living at the end of treatment relative to placebo-arm subjects (n = 17). Notably, these gains were still evident in full at the 4 week follow-up and in part at the 20 week follow up [figure 1]. Outcomes were obtained with excellent treatment blinding and high treatment adherence/participant satisfaction.
Conclusions: Daily use of the CVS device may provide a feasible and effective adjunct therapy for the management of symptoms associated with PD. A fully-powered, multi-site effectiveness study is needed to replicate effect and further assess durability. Parallel mechanistic studies are needed to isolate physiological effect and optimise dose.
References: (1) Wilkinson D, et al. NeuroRehabilitation 2016; 38:179-182. (2) Black R, et al. IEEE Journal of Translational Engineering in Heath and Medicine 2016; 4:10.
To cite this abstract in AMA style:
K. Ade, A. Podlewska, T. Pellet-Higgins, S. Banducci, M. Bodani, M. Sakel, D. Wilkinson. Feasibility and Efficacy of Brainstem Modulation Therapy for the Management of Parkinson’s Disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/feasibility-and-efficacy-of-brainstem-modulation-therapy-for-the-management-of-parkinsons-disease/. Accessed October 17, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/feasibility-and-efficacy-of-brainstem-modulation-therapy-for-the-management-of-parkinsons-disease/