Objective: To assess QTc prolongation risk of deutetrabenazine and its active metabolites by conducting exposure-QTc interval modeling, and to predict QTc prolongation in both CYP2D6 extensive and poor metabolizers at clinical exposure.
Background: Deutetrabenazine is a deuterated form of tetrabenazine indicated for chorea associated with Huntington’s disease and tardive dyskinesia. The deuterium isotope effect attenuates the rate of CYP2D6-catalyzed metabolism of the active metabolites (α- and β-HTBZ), increasing half-life and achieving similar exposure for lower doses compared to tetrabenazine. In a phase 1 study, escalating single doses of deutetrabenazine or placebo were administered to healthy volunteers who were either CYP2D6 extensive/intermediate metabolizers (EM) or poor metabolizers (PM) to determine plasma concentrations of parent and active metabolites and collect corresponding ECG data for concentration-QTc modeling.
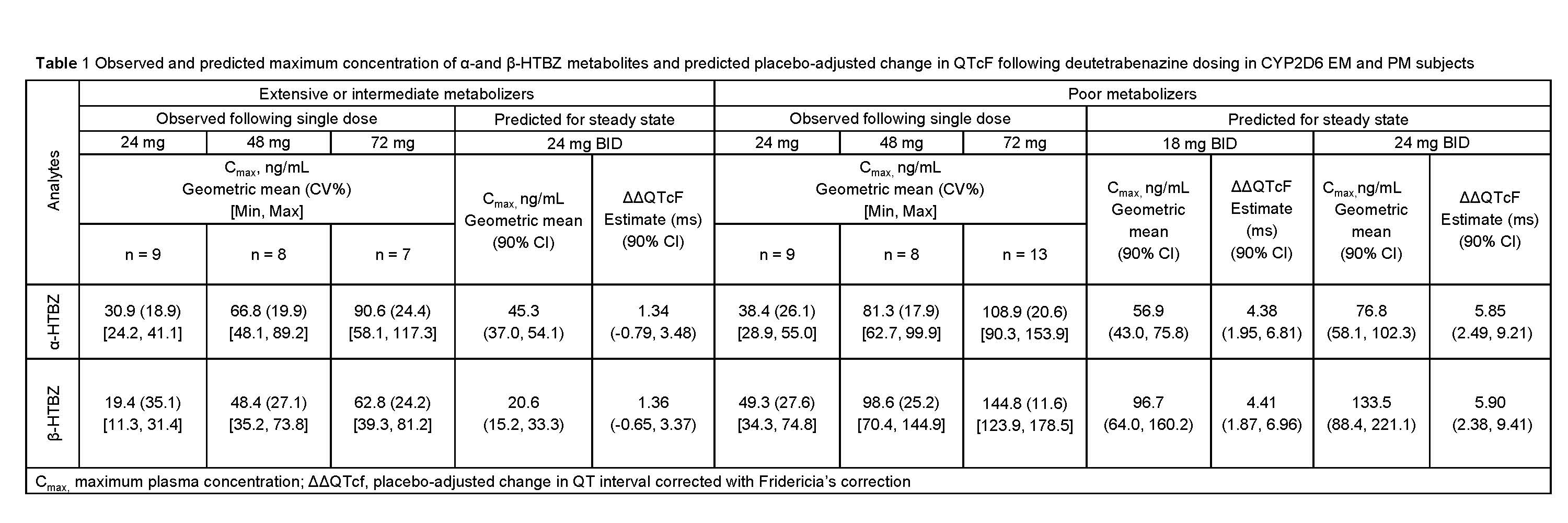
Method: Each EM n=12 and PM n=12 subject received a sequence of deutetrabenazine doses (24mg, 48mg and 72mg) or placebo (treatments separated by 7 days) and additional PM subjects received 72mg n=9 or placebo n=3. Following dosing, 18 PK blood samples were obtained over 72 hours and were time-matched with replicate 12-lead ECGs extracted from continuous ECG recordings for up to 24 hours. A linear mixed-effects concentration-QTc modeling (C-QTc) analysis characterized the relationship between plasma concentrations of deutetrabenazine and α-HTBZ and β-HTBZ metabolites, and ΔQTcF (change from baseline in QTcF).
Results: Metabolite exposures exceeding the highest concentrations predicted for the 24mg BID dose were attained in both EM and PM groups [Table 1]. Following exploration of a full model for the C-QTc analysis including all analytes (parent, α-HTBZ, and β-HTBZ) and reduced models, a primary model with α-HTBZ and β-HTBZ was selected. Based on the C-QTc analysis of pooled EM and PM data, a clinically relevant effect of deutetrabenazine on the placebo-adjusted Δ QTcF > 10 msec can be excluded within the observed range of α-HTBZ and β-HTBZ plasma concentrations up to 150000 pg/mL and 140000 pg/mL. Based on this analysis, an effect exceeding 10 msec can be excluded at the maximum recommended doses in both populations.
Conclusion: For extensive/intermediate and poor CYP2D6 metabolizers, deutetrabenazine treatment does not have a clinically relevant effect on QT prolongation at maximum recommended doses.
To cite this abstract in AMA style:
F. Schneider, B. Darpo, M. Gutierrez, M. Gordon, L. Rabinovich-Guilatt. Evaluation of the QTc prolongation risk of deutetrabenazine [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/evaluation-of-the-qtc-prolongation-risk-of-deutetrabenazine/. Accessed April 20, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/evaluation-of-the-qtc-prolongation-risk-of-deutetrabenazine/