Session Information
Date: Thursday, June 8, 2017
Session Title: Parkinson's Disease: Neuroimaging And Neurophysiology
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: To evaluate [18F]MNI-944, a highly selective synaptic SV2A PET imaging tracer, as a biomarker for longitudinal studies in early PD.
Background: PD symptoms manifest from loss of specific neuronal populations and resultant regional reductions in synaptic density may reflect various motor or cognitive deficits. Additionally, long-term motor complications from denervation and dopaminergic therapy may arise in part from increased striatal synaptic density. SV2A (Synaptic vesicle glycoprotein 2A), the target of the anti-epileptic levetiracetam, is a constitutive synaptic vesicle protein. Thus, regional SV2A density reflects synaptic density. Recent studies with a11C-labeled SV2A PET tracer have confirmed signal correlates with known SV2A loss in temporal lobe epilepsy. Due to the longer half-life of 18F, an 18F-labeled SV2A PET tracer confers substantial advantages, such as distribution for multi-site clinical trials.
Methods: [18F]MNI-944 PET imaging and blood metabolism were validated in non-human primate (NHP) and compared with previously reported studies with [11C]UCB-J. Imaging was acquired starting immediately post-injection and regional standard uptake volumes (SUV) determined. Human subjects include HC and subjects with PD, including PD with motor complications.
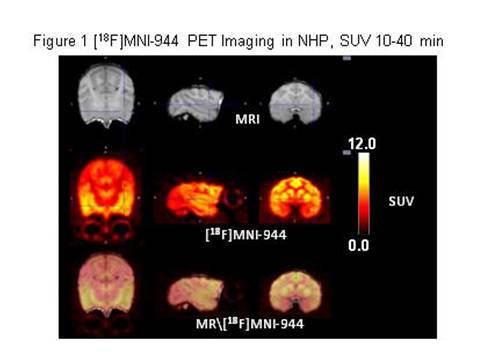
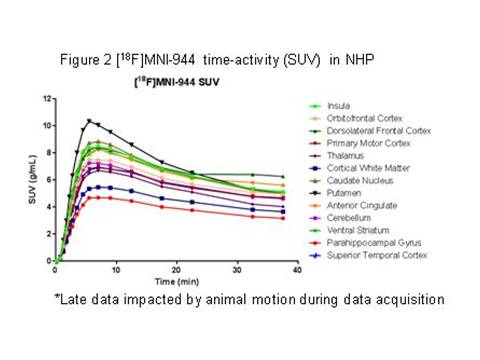
Results: In NHP, blood metabolism of [18F]MNI-944 was similar to [11C]UCB-J and no significant metabolites were identified. Brain penetration, regional uptake, and washout for [18F]MNI-944 were also highly comparable. Imaging post-injection demonstrated high [18F]MNI-944 uptake in all cortical and cerebellar grey matter areas, as well as striatum, as expected for known SV2A distribution (see Figures 1 [figure1] and 2 [figure2] ).
Conclusions: Validation studies demonstrate excellent characteristics of [18F]MNI-944 PET as an SV2A imaging biomarker, enabling investigations of alterations in synaptic density in humans. Data will be presented comparing HC to early and late PD, with emphasis on striatal and frontal cortical regions.
References: 1. Brotchie JM. “Nondopaminergic Mechanisms in Levodopa-Induced Dyskinesia.” Movement Disorders, (2005) 20(8):919–931.
2. Finnema SJ, Nabeel B. Nabulsi NB,Eid T, et al., “Imaging synaptic density in the living human brain.” Science Translational Medicine 8 (348), 348ra96.
To cite this abstract in AMA style:
D. Russell, D. Alagille, V. Carroll, M. Thompson, J. Madonia, O. Barret, C. Constantinescu, J. Seibyl, K. Marek, G. Tamagnan. Evaluation of SV2A expression with [18F]MNI-944 PET imaging in healthy controls (HC) and Parkinson disease (PD) [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/evaluation-of-sv2a-expression-with-18fmni-944-pet-imaging-in-healthy-controls-hc-and-parkinson-disease-pd/. Accessed December 24, 2025.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/evaluation-of-sv2a-expression-with-18fmni-944-pet-imaging-in-healthy-controls-hc-and-parkinson-disease-pd/