Category: Ataxia
Objective: To compare the clinical efficacy of different pharmacological agents in reduction of disease severity of Friedreich Ataxia.
Background: Friedreich ataxia is a rare, inherited, progressive neurodegenerative disorder, with a global prevalence of 1 in every 22,000 to 50,000 people. The ambiguity surrounding the best pharmacological treatment necessitates a thorough review of recent evidence to identify and compare effective management approaches.
Method: We conducted a systematic search across various databases, including PubMed/MEDLINE, Cochrane, SCOPUS, Embase, and Clinicaltrials.gov, to identify RCTs addressing interventions for Friedrich ataxia. This search spanned from the earliest records to November 2023. We performed pairwise and network meta-analyses to compare interventions directly and indirectly, quantifying the results as mean differences (MD).
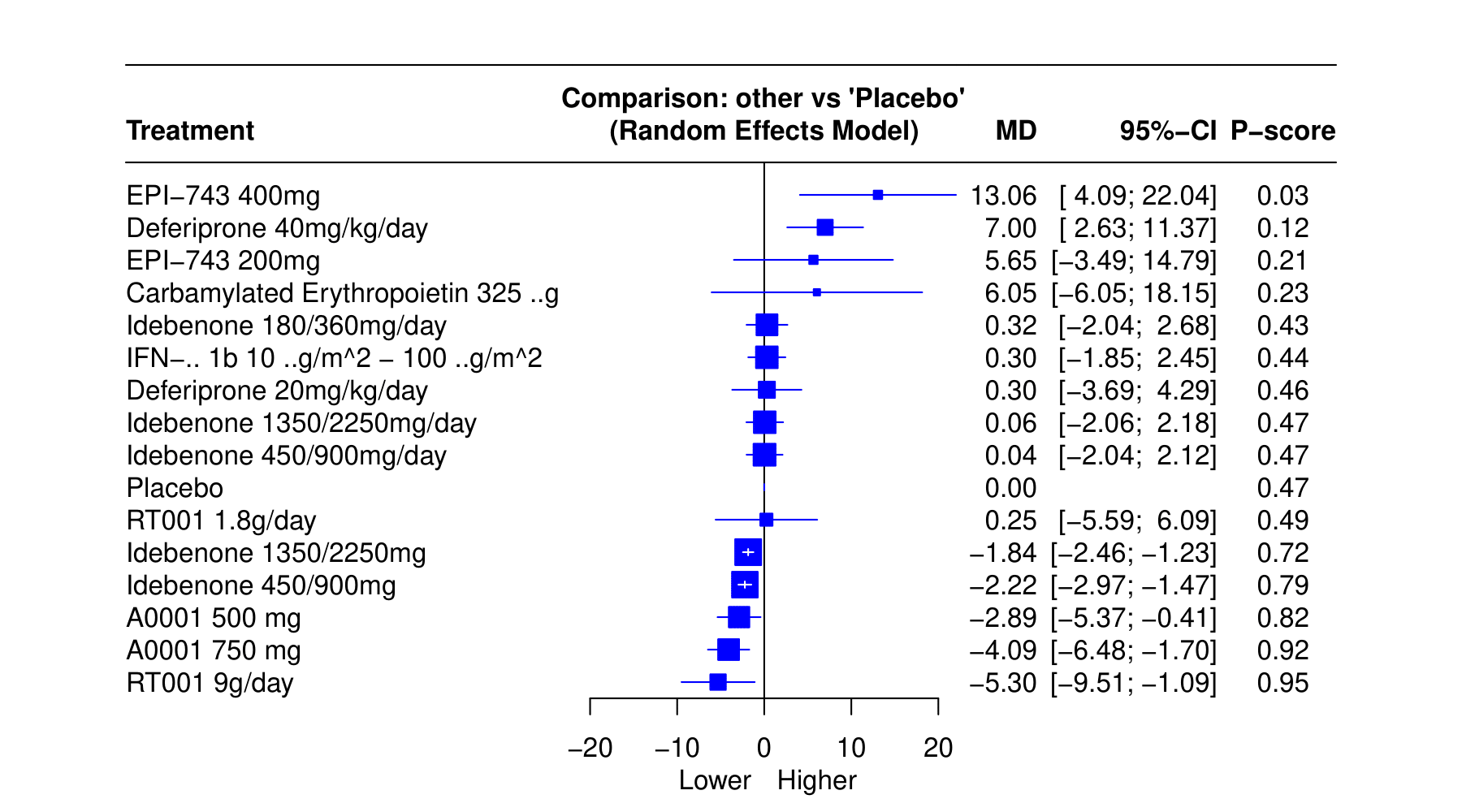
Results: We included 16 RCTs with 1964 patients, assessing effectiveness of 14 different interventions. The mean difference (MD) in Functional Assessment Rating Scale (FARS) scores between RT001 9g/day and placebo demonstrated a MD of −5.30 (95% CI: −9.51; −1.09, p = 0.95). A0001 750 mg and A0001 500 mg had MD of −4.09 (95% CI: −6.48; −1.70, p = 0.92) and −2.89 (95% CI: −5.37; −0.41, p = 0.82) respectively. Different doses of idebenone showed varying effects on FARS scores. Idebenone 450/900mg/day and Idebenone 1350/2250mg/day exhibited significant improvements, with MDs of -2.22 (95% CI: -2.97; -1.47, p = 0.79) and -1.84 (95% CI: -2.46; -1.23, p = 0.72) respectively. Conversely, Idebenone 180/360 mg/day did not show significant differences compared to placebo 0.32 (95% CI: −2.04; 2.68, p = 0.43). EPI-743 400mg and Deferiprone 40mg/kg/day demonstrated a poor response with MD of 13.06 (95% CI: 4.09; 22.04, p = 0.03) and 7.00 (95% CI: 2.63; 11.37, p = 0.12) respectively. Conversely, EPI-743 200mg and Carbamylated Erythropoietin 325μg did not show significant differences compared to placebo, with MDs of 5.65 (95% CI: -3.49; 14.79, p = 0.21) and 6.05 (95% CI: -6.05; 18.15, p = 0.23) respectively. Additionally, IFN-1b 10μg/m^2 – 100μg/m^2 and Deferiprone 20mg/kg/day demonstrated minimal changes with MDs of 0.30 (95% CI: -1.85; 2.45, p = 0.44) and 0.30 (95% CI: -3.69; 4.29, p = 0.46) respectively.
Conclusion: Our preliminary analysis shows RT001 9g/day, A0001 750mg, and Idebenone variations yield significant improvements in FA patients.
Forest Plot
To cite this abstract in AMA style:
T. Dave, V. Kumar, R. Raj, MA. Shamim, V. Suresh, K. Patel, M. Hassan, A. Bhonsale, DVV. Krishna, B. Dhakal, A. Ambulinambi, D. Dey. Evaluating Pharmacological Interventions for Friedreich Ataxia: A Network Meta-Analysis of Randomised Trials [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/evaluating-pharmacological-interventions-for-friedreich-ataxia-a-network-meta-analysis-of-randomised-trials/. Accessed December 25, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/evaluating-pharmacological-interventions-for-friedreich-ataxia-a-network-meta-analysis-of-randomised-trials/