Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To evaluate the real-world long-term effectiveness of CLES on advanced PD patients and their caregivers.
Background: Carbidopa/levodopa enteral suspension (CLES) was approved in USA for the treatment of motor fluctuations in patients with advanced Parkinson’s Disease (PD) in 2015. While previous clinical studies have shown the short and medium term impact of CLES on motor symptoms and non-motor symptoms1,2 , long-term data reflective of the real-world clinical practice in USA is emerging.
Methods: PROviDE is a prospective, longitudinal observational study to evaluate long-term effectiveness of CLES in advanced PD patients and their caregivers. All patients participating in the patient support program are eligible for the study. The study participants complete a battery of validated patient-reported outcomes at baseline and periodically up to 36 months after treatment initiation. The primary outcome of the study is change in self-reported off-time compared to baseline. Secondary outcomes include: quality of life (PDQ-8), sleep (PDSS-2), treatment satisfaction (TSQM), freezing of gait (FOGQ-sa), impact of dyskinesia on activity of daily living (PDYS-26), fatigue (PROMIS), global impression of improvement (PGIC) and health economic outcomes. Caregiver outcomes include quality of life (PDQ-Carer), work productivity (WPAI) and treatment satisfaction. In a sub-study, motor symptoms are also being evaluated using a validated wearable sensor system.
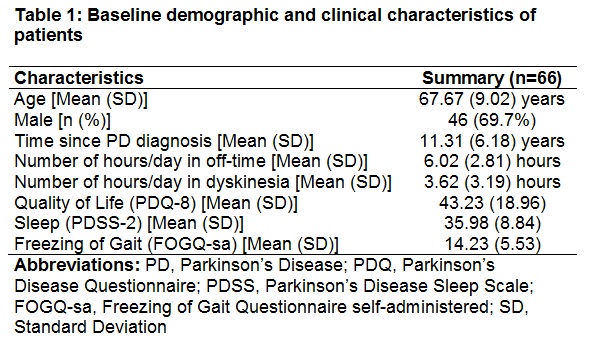
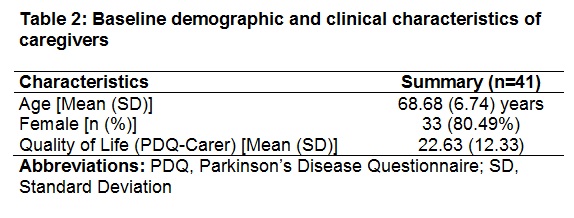
Results: The enrollment for PROviDE study is ongoing (anticipated end of enrollment ~ Q3 2018). The baseline demographic and clinical characteristics are presented for currently enrolled patients (n=66) [Table 1] and caregivers (n=41) [Table 2]. The baseline characteristics of patients enrolled in PROvoiDE study are similar to CLES clinical trials1,2. About 15% (n=10) of the patients reported to being on DBS during baseline.
Conclusions: The PROviDE study is a proof-of-concept demonstrating the feasibility of conducting an observational study on patients enrolled in support programs to evaluate the real-world effectiveness orphan drugs. Inclusion of all patients, while adding heterogeneity, increases the generalizability of results to the real-world clinical practice.
References: 1 Olanow WC et al. (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study.” Lancet Neurol; 13(2):141-149. 2 Standaert DG et al. (2017) Effect of levodopa-carbidopa intestinal gel on non-motor symptoms in patients with advanced Parkinson disease. Mov Disord Clin Pract; 4(6):829-837.
To cite this abstract in AMA style:
R. Pahwa, R. Dorsey, P. Kandukuri, Y. Bao, J. Zamudio, I. Pan, Y. Jalundhwala. Evaluating long-term effectiveness of carbidopa/levodopa enteral suspension in advanced Parkinson’s Disease patients: PROviDE study design and baseline characteristics [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/evaluating-long-term-effectiveness-of-carbidopa-levodopa-enteral-suspension-in-advanced-parkinsons-disease-patients-provide-study-design-and-baseline-characteristics/. Accessed December 22, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/evaluating-long-term-effectiveness-of-carbidopa-levodopa-enteral-suspension-in-advanced-parkinsons-disease-patients-provide-study-design-and-baseline-characteristics/