Category: Parkinson’s Disease: Clinical Trials
Objective: We aimed to assess whether clonidine, a specific α2-adrenergic receptor agonist, would improve impulse controls disorders (ICDs).
Background: ICDs are frequently encountered in Parkinson’s disease (PD) with a 5‐year cumulative incidence rate of 46% (1).
Method: We conducted a multicentre trial in five French movement disorder departments. Patients with PD and ICDs (n=41) were enrolled in an 8-week, randomised (1:1), double blind, placebo-controlled study of clonidine (75 mg twice a day). Randomisation and allocation to trial group were carried out by a central computer system. The primary outcome was the change at 8 weeks in symptom severity using the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease–Rating Scale (QUIP-RS) score. A reduction of the most elevated subscore of the QUIP-RS of more than 3 points without any increase in the other QUIP-RS dimension defined success. If the one-sided 90% CI contains 15%, a phase 3 study could be considered. The key secondary outcome measure was the modification of total QUIP-RS score at 8 weeks.
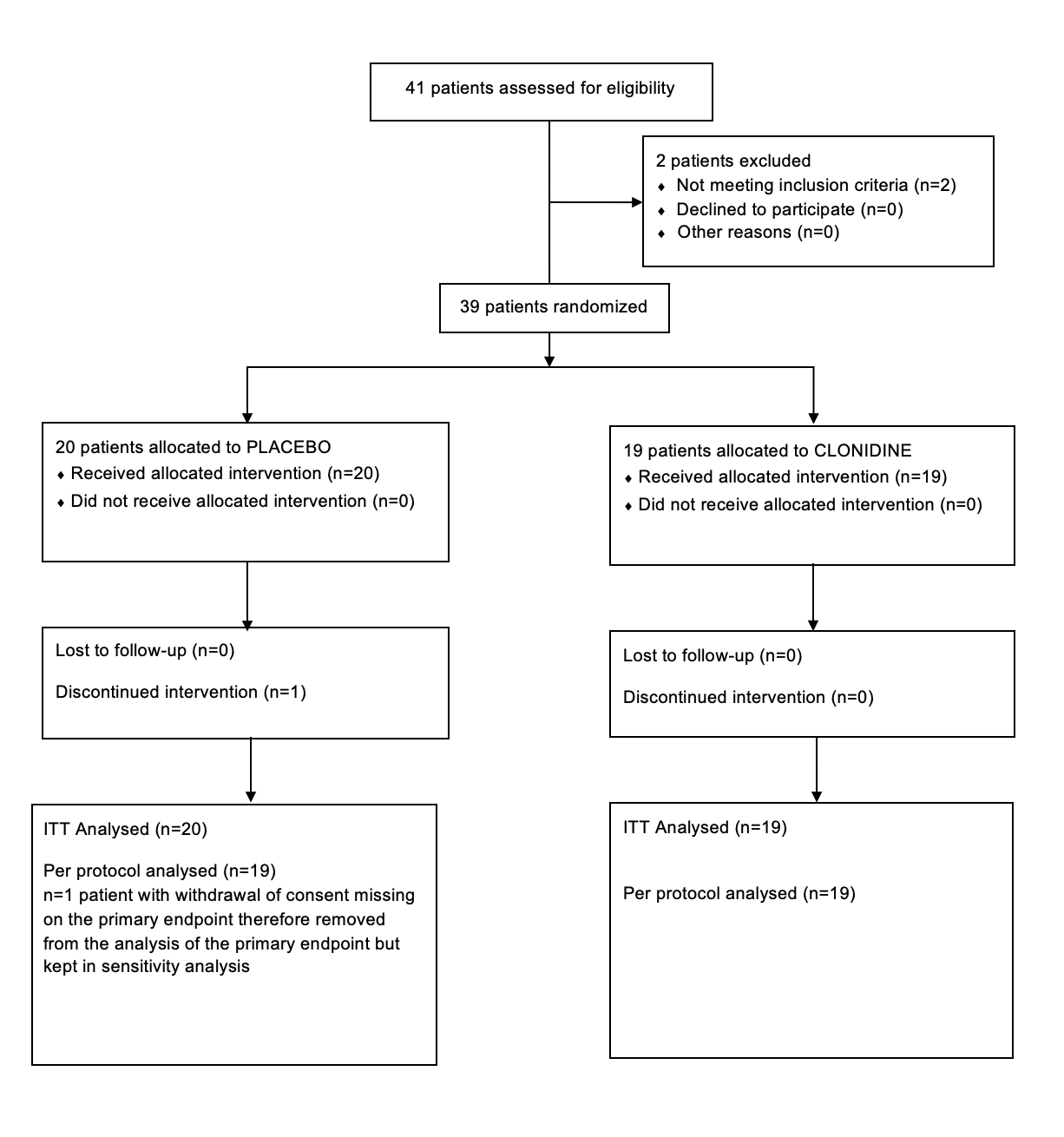
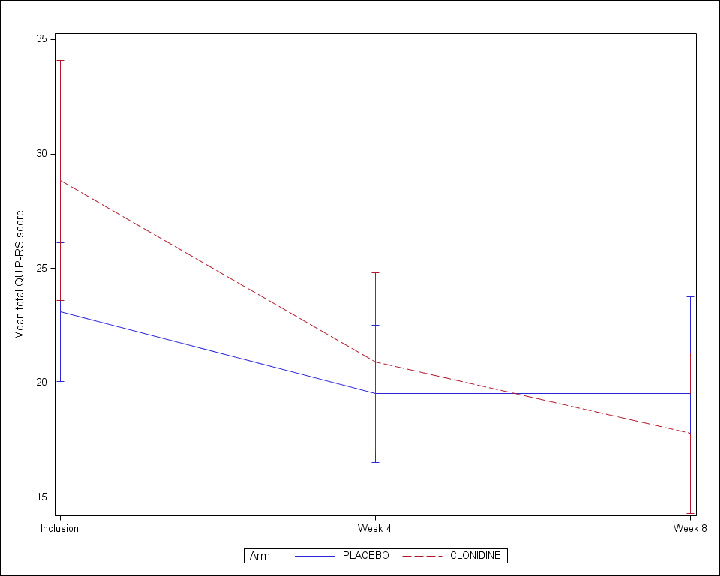
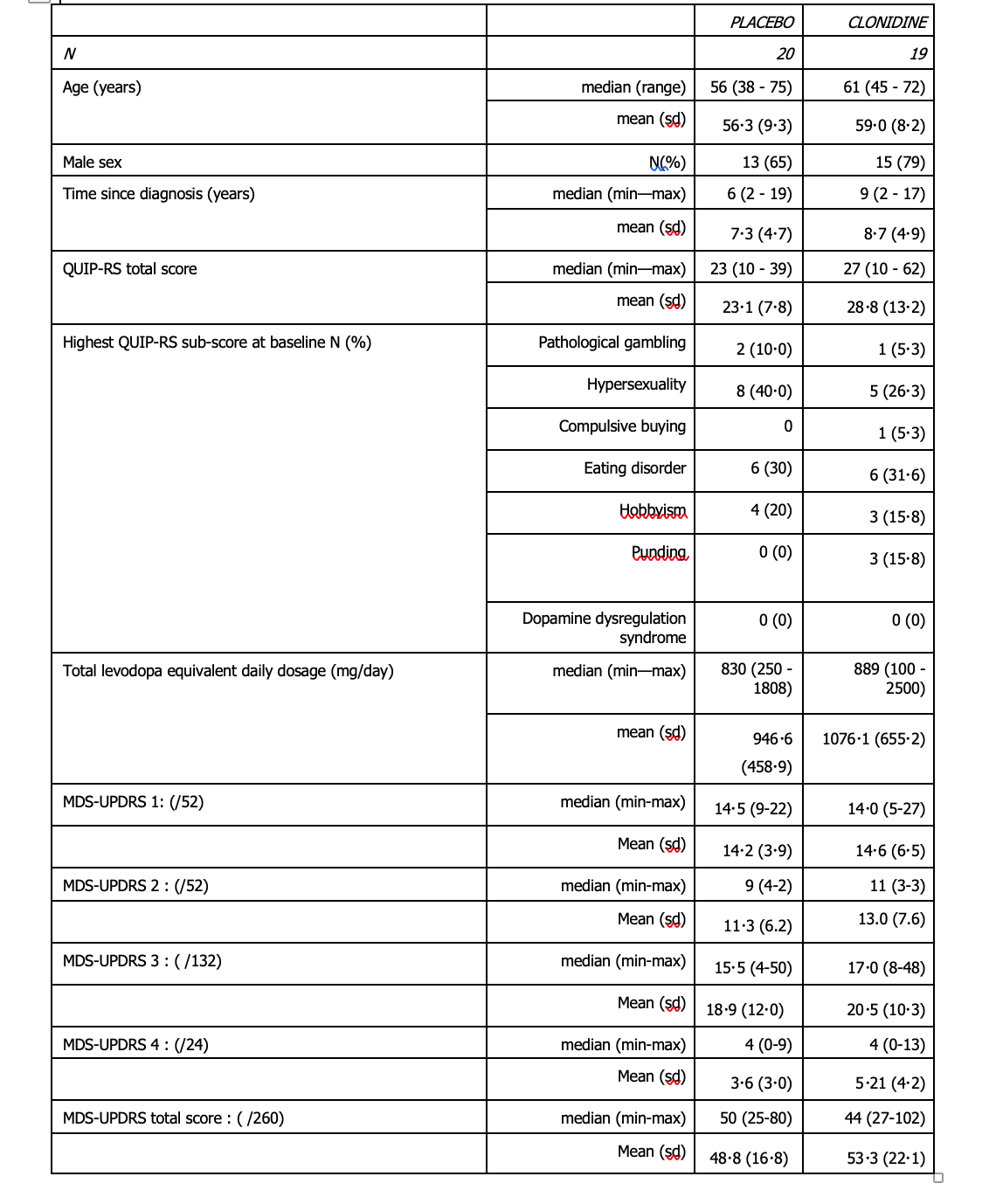
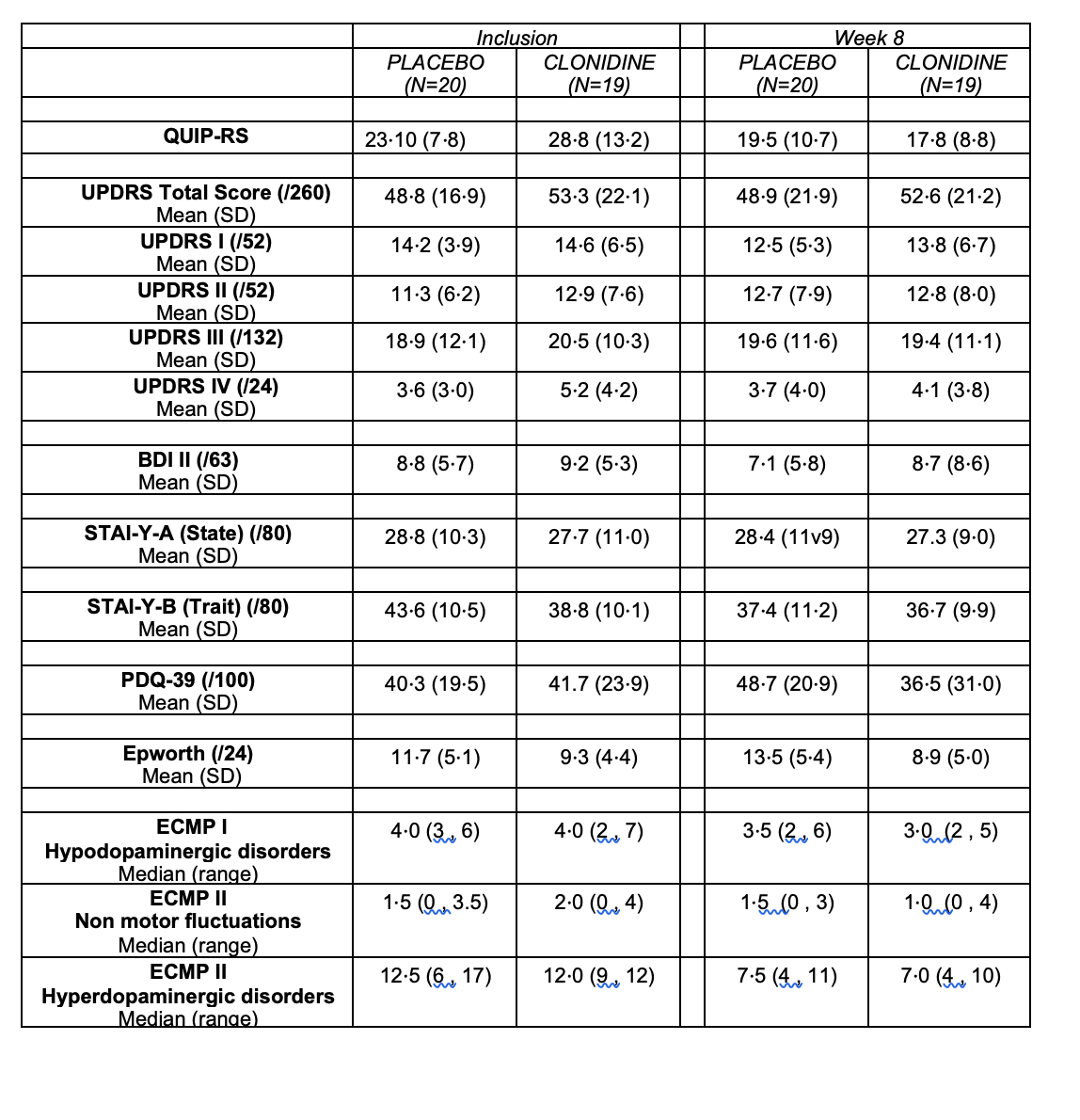
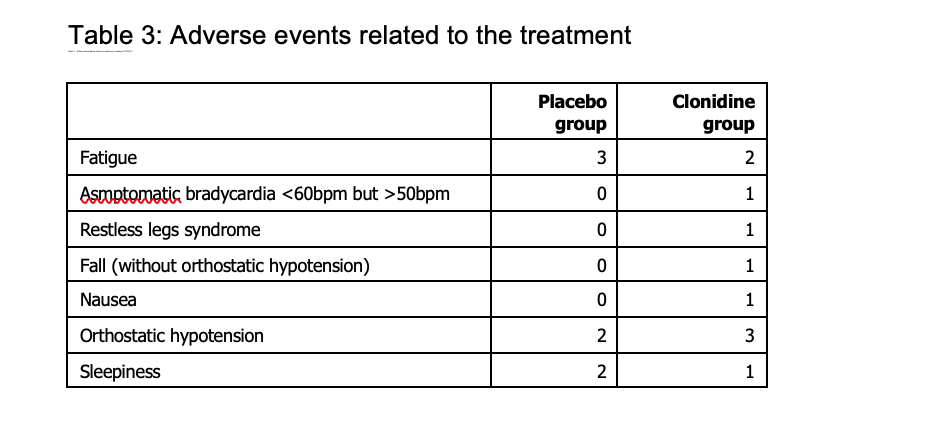
Results: Between 15 May 2019 and 10 September 2021, 19 patients in the clonidine group and 20 patients in the placebo group were enrolled (figure 1, table1). The proportion difference of success in reducing QUIP-RS at 8 weeks, was 7% (one-sided upper 90% CI: 27%) with 42.1% of success in the clonidine group and 35.0 % in the placebo group. Compared to patients in the placebo group, patients in the clonidine group experienced a greater reduction in the total QUIP-RS score at 8 weeks (reduction of 11.0 points vs. 3.6) (figure 2 and table 2). Asymptomatic orthostatic hypotension was observed in three patients under clonidine (table 3).
Conclusion: Clonidine was well tolerated but our study was not enough powerful to demonstrate significant superiority compared to placebo in reducing ICDs despite a greater reduction of total QUIP score at 8 weeks. A phase 3 study should be conducted.
References: Corvol JC, Artaud F, Cormier-Dequaire F, Rascol O, Durif F, Derkinderen P, et al. Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology. 2018 Jul 17;91(3):e189–201.
To cite this abstract in AMA style:
C. Laurencin, A. Marques, D. Dilly Duchez, C. Giordana, S. Meoni, M. Anheim, P. Boulinguez, B. Ballanger, S. Thobois. Efficacy and safety of clonidine for the treatment of impulse control disorder in Parkinson’s disease: a multicentre, parallel, randomised, double blind, phase 2b clinical trial [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-clonidine-for-the-treatment-of-impulse-control-disorder-in-parkinsons-disease-a-multicentre-parallel-randomised-double-blind-phase-2b-clinical-trial/. Accessed December 22, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-clonidine-for-the-treatment-of-impulse-control-disorder-in-parkinsons-disease-a-multicentre-parallel-randomised-double-blind-phase-2b-clinical-trial/