Category: Surgical Therapy: Parkinson's Disease
Objective: To investigate short-term effects of combined deep brain stimulation (DBS) of the substantia nigra pars reticulata (SNr) and subthalamic nucleus (STN) on gait performance, turning and transitions and map corresponding stimulation volumes within the STN and SNr.
Background: The SNr has been proposed as an alternative target for axial symptoms, gait impairment and freezing of gait in patients with Parkinson’s disease. As SNr DBS alone is not efficient on segmental symptoms, paradigms of combined STN and SNr stimulation have recently been suggested.[1]
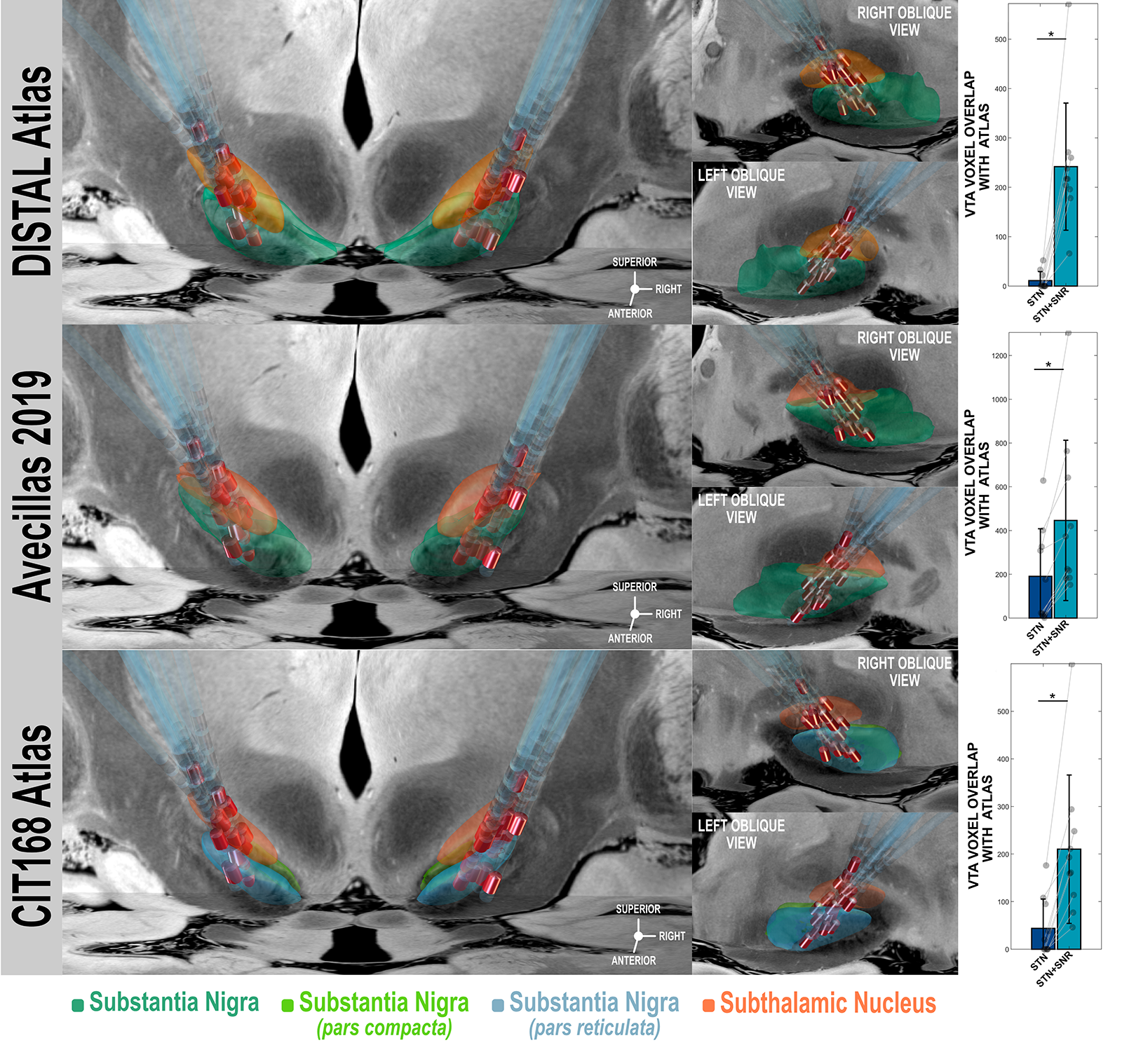
Method: 10 patients were included for which properties of implanted DBS electrodes allowed co-stimulation of SNr via ventral contacts. In a double-blinded crossover design, clinical scores and kinematic parameters were obtained during walking and turning tasks with STN DBS, combined STN-SNr DBS and OFF DBS, each within 30mins after reprogramming. Gait assessments were conducted with a commercial system using inertial sensors. DBS-electrodes and active contacts were mapped to a common space, stimulation volumes were modeled and voxel overlaps with three different normative atlases of the SNr were computed [2].
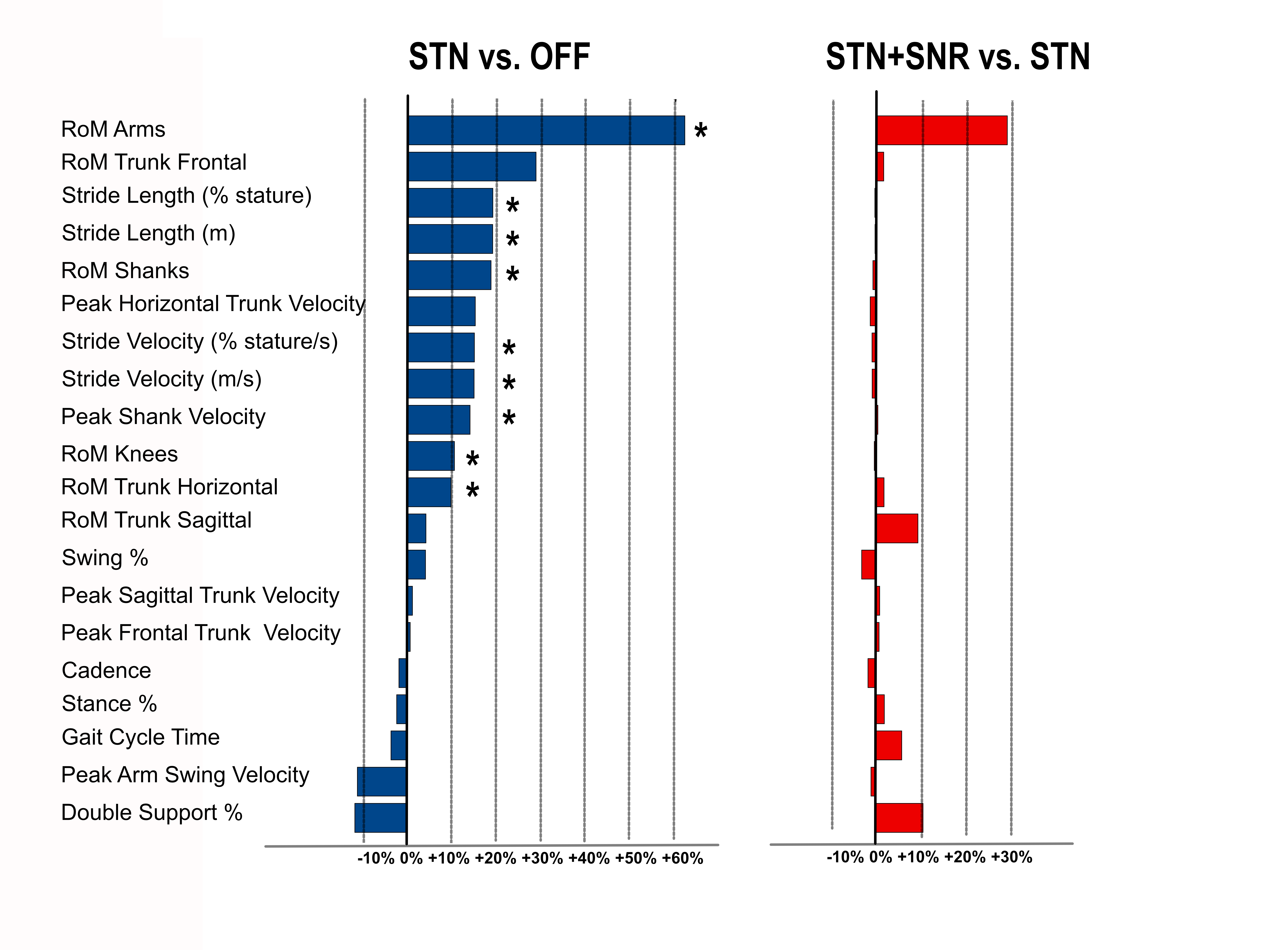
Results: STN DBS led to significant improvements (all p<0.05) of MDS-UPDRS III (-42%), stride length (+19%), stride velocity (+15%), ranges of motion of shanks (+18%), knees (+11%), arms (+62%) and trunk in horizontal plane (+10%), peak velocity of shanks (+14%), turn duration (-22%) and peak velocity (+27%) as well as peak velocities during transitions (+41%) and range of motion of trunk during sit-to-stand (+30%) when compared to OFF. For none of these parameters, an additional significant effect could be detected with combined STN-SNr DBS. (Figure 1). In all patients, stimulation volumes of combined STN-SNr DBS were overlapping with a significantly larger number of voxels (all p<0.05) of all atlases of SNr compared to STN-DBS alone. (Figure 2)
Conclusion: Despite robust verification of stimulation volumes overlapping with SNr, no additional benefit of short term, combined high frequency DBS of STN and SNr could be observed in this cohort. With the approach used here, DBS of SNr remains confined to the dorsolateral portion of the SNr. Further confounders may be inadequate duration of stimulation intervals, inappropriate stimulation frequency and patient selection for target symptoms.
References: 1. Weiss, D., et al., Nigral stimulation for resistant axial motor impairment in Parkinson’s disease? A randomized controlled trial. Brain, 2013. 136(Pt 7): p. 2098-108.
2. Horn, A., et al., Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage, 2019. 184: p. 293-316.
To cite this abstract in AMA style:
D. Kroneberg, B. Al-Fatly, C. Morkos, GH. Schneider, A. Kühn. Effects of combined subthalamic and dorsolateral nigral deep brain stimulation on gait performance in Parkinson’s Disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/effects-of-combined-subthalamic-and-dorsolateral-nigral-deep-brain-stimulation-on-gait-performance-in-parkinsons-disease/. Accessed February 6, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/effects-of-combined-subthalamic-and-dorsolateral-nigral-deep-brain-stimulation-on-gait-performance-in-parkinsons-disease/