Objective: To examine the effect of CLES at 12 months of follow-up in APD patients with moderate to severe fatigue.
Background: Fatigue, one of the most disabling symptoms of PD, is prevalent in about 82% of APD patients [1]. It is significantly associated with sleep dysfunction, low physical activity, poor quality of life and is the most common reason for work disability and need for social security disability benefits among PD patients [2]. CLES has shown significantly greater reduction in fatigue than sub-cutaneous apomorphine infusion, while the prevalence of fatigue after DBS surgery increases and is a long-term side effect [3-5].
While previous studies have demonstrated the effectiveness of CLES in improving fatigue in APD patients, there is a lack of understanding of impact of CLES on overall outcomes in patients with greater fatigue.
Method: PROviDE is a longitudinal, observational home-based study, which follows APD patients treated with CLES for 3 years to evaluate patient-reported outcomes including fatigue [6]. Fatigue was measured using PROMIS Short Form v1.0 – Fatigue 8a which has a standardized mean of 50 [7]. Patients with baseline T-scores ≥ 60 were considered to have moderate to severe fatigue [8,9]. Unlike NMSS, PROMIS Fatigue measures fatigue independently of sleep. In this 12-month interim sub-group analysis, we examined the effect of CLES on fatigue and patient reported outcomes such as off-time, PDSS-2, FOGQ-sa, feeling of unsteadiness, PDQ-8, and treatment satisfaction.
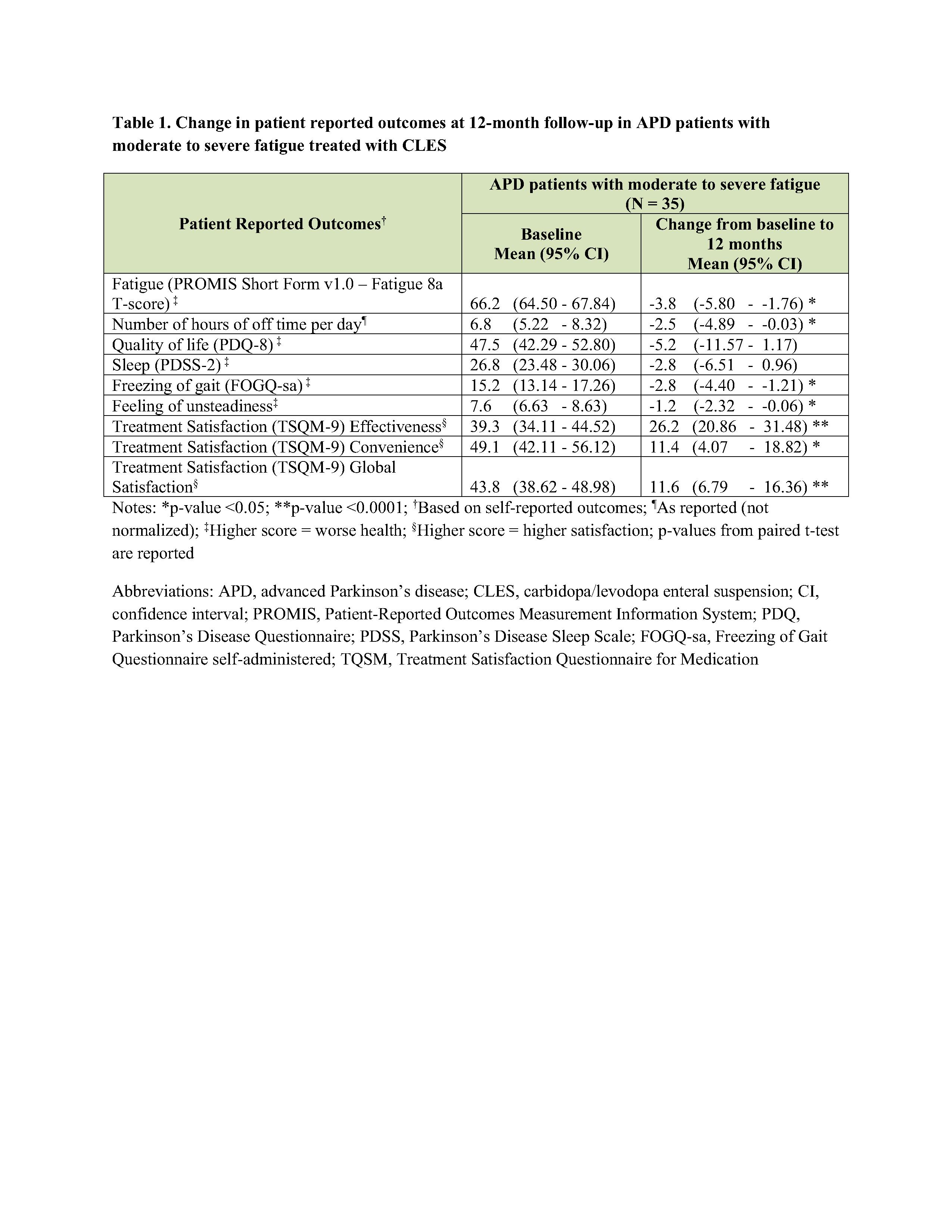
Results: In this sub-group analysis, 35 (42.2%; mean age, 68.5 [SD=7.6]) patients had moderate to severe fatigue at baseline. At the 12-month follow-up, there was significant reduction in fatigue (mean: -3.78; CI: -5.80, -1.76; p-value <0.05). Significant reduction was also observed in the number of hours of off time per day, freezing of gait, and feeling of unsteadiness; and significant improvement in treatment satisfaction effectiveness, convenience, and global satisfaction domains [Table 1]. This sub-group also demonstrated numeric reduction in PDSS-2 sleep scores and PDQ-8 quality of life scores.
Conclusion: APD patients with moderate to severe fatigue treated with CLES showed significant improvements in fatigue, off-time, and other patient reported outcomes. CLES is a viable option benefitting patients with moderate to severe fatigue.
References: 1. Barone, P., Antonini, A., Colosimo, C., Marconi, R., Morgante, L., Avarello, T. P., … & Cicarelli, G. (2009). The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society, 24(11), 1641-1649. 2. Zesiewicz, T. A., Patel-Larson, A., Hauser, R. A., & Sullivan, K. L. (2007). Social security disability insurance (SSDI) in Parkinson’s disease. Disability and Rehabilitation, 29(24), 1934-1936. 3. Martinez‐Martin, P., Reddy, P., Katzenschlager, R., Antonini, A., Todorova, A., Odin, P., … & Bryndum, N. (2015). Euro I nf: AM ulticenter C omparative O bservational S tudy of A pomorphine and L evodopa I nfusion in P arkinson’s D isease. Movement Disorders, 30(4), 510-516. 4. Kluger, B. M., Parra, V., Jacobson, C., Garvan, C. W., Rodriguez, R. L., Fernandez, H. H., … & Okun, M. S. (2012). The prevalence of fatigue following deep brain stimulation surgery in Parkinson’s disease and association with quality of life. Parkinson’s Disease, 2012. 5. Lilleeng, B., Gjerstad, M., Baardsen, R., Dalen, I., & Larsen, J. P. (2015). The long‐term development of non‐motor problems after STN‐DBS. Acta Neurologica Scandinavica, 132(4), 251-258. 6. Pahwa R. Evaluating long-term effectiveness of carbidopa/levodopa enteral suspension in advanced Parkinson’s Disease patients: PROviDE study design and baseline characteristics. Movement disorders. 2018;33:S93. 7. Cella, D., Lai, J. S., Jensen, S. E., Christodoulou, C., Junghaenel, D. U., Reeve, B. B., & Stone, A. A. (2016). PROMIS fatigue item bank had clinical validity across diverse chronic conditions. Journal of clinical epidemiology, 73, 128-134. 8. Cella, D., Choi, S., Garcia, S., Cook, K. F., Rosenbloom, S., Lai, J. S., … & Gershon, R. (2014). Setting standards for severity of common symptoms in oncology using the PROMIS item banks and expert judgment. Quality of Life Research, 23(10), 2651-2661. 9. Noonan, V. K., Cook, K. F., Bamer, A. M., Choi, S. W., Kim, J., & Amtmann, D. (2012). Measuring fatigue in persons with multiple sclerosis: creating a crosswalk between the Modified Fatigue Impact Scale and the PROMIS Fatigue Short Form. Quality of Life Research, 21(7), 1123-1133.
To cite this abstract in AMA style:
S. Isaacson, J. Aldred, D. Cella, P. Kandukuri, N. Gupta, Y. Jalundhwala, P. Kukreja, I. Pan, R. Pahwa. Effectiveness of Carbidopa/Levodopa Enteral Suspension (CLES) in Advanced Parkinson’s Disease (APD) Patients with Moderate to Severe Fatigue: Sub-group Analysis from PROviDE study [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/effectiveness-of-carbidopa-levodopa-enteral-suspension-cles-in-advanced-parkinsons-disease-apd-patients-with-moderate-to-severe-fatigue-sub-group-analysis-from-provide-study/. Accessed December 25, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/effectiveness-of-carbidopa-levodopa-enteral-suspension-cles-in-advanced-parkinsons-disease-apd-patients-with-moderate-to-severe-fatigue-sub-group-analysis-from-provide-study/