Session Information
Date: Monday, September 23, 2019
Session Title: Genetics
Session Time: 1:45pm-3:15pm
Location: Les Muses Terrace, Level 3
Objective: To investigate the longitudinal association between PD-associated polygenic load and the striatal dopaminergic activity using 123I-N-3-fluoropropyl-2-beta-carboxymethoxy-3beta-(4-iodophenyl) nortropane (123I-FP-CIT) single photon emission computed tomography (SPECT).
Background: Recent genome-wide association studies (GWASs) have shown that a number of single nucleotide polymorphisms (SNPs) in several genes are associated with an increased susceptibility toward PD. However, the relationship between polygenic load and striatal dopaminergic degeneration in PD remains still unclear.
Method: Using data from 335 PD patients in the Parkinson’s Progression Markers Initiative (PPMI) database, we investigated the longitudinal association of PD-associated polygenic load with changes in striatal dopaminergic activity as measured by 123I-N-3-fluoropropyl-2-beta-carboxymethoxy-3beta-(4-iodophenyl) nortropane (123I-FP-CIT) single photon emission computed tomography (SPECT) over 4 years. PD-associated polygenic load was estimated by calculating weighted genetic risk scores (GRS) using: i) all available 27 PD-risk single nucleotide polymorphisms (SNPs) in the PPMI database (GRS1); and ii) 23 SNPs with minor allele frequency > 0.05 (GRS2).
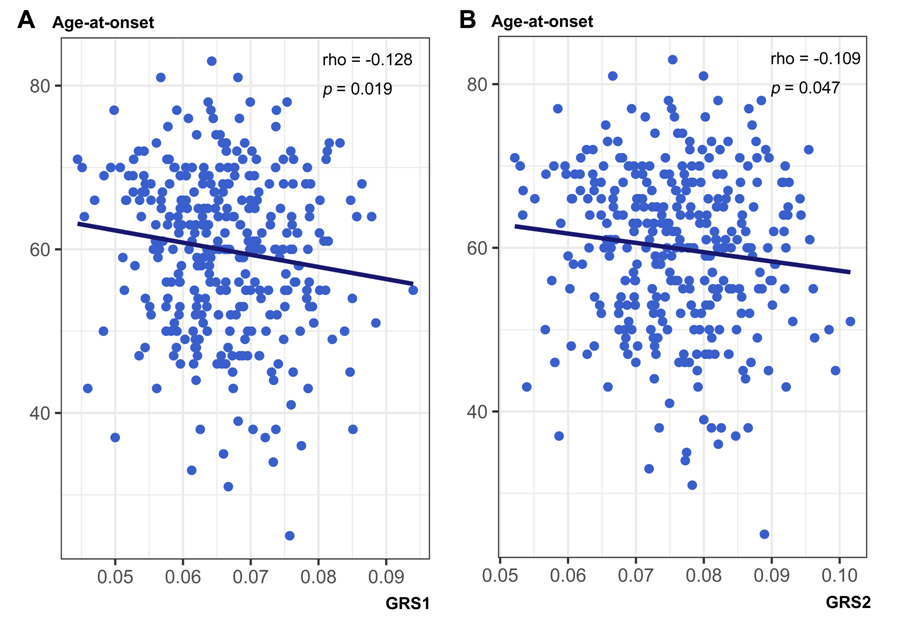
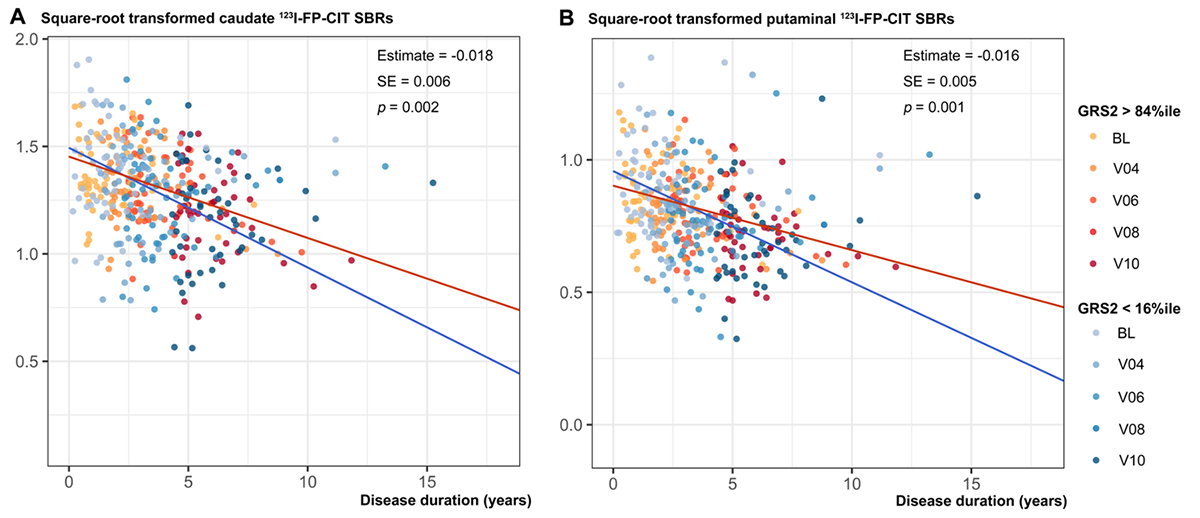
Results: GRS1 and GRS2 were correlated with younger age-at-onset in PD patients (GRS1, Spearman’s rho = -0.128, p = 0.019; GRS2, Spearman’s rho = -0.109, p = 0.047; Figure 1). Although GRS1 did not show an association with changes in striatal 123I-FP-CIT availability, GRS2 was associated with a slower decline of striatal dopaminergic activity (interactions with disease duration in linear mixed model; caudate nucleus, estimate = 0.399, SE = 0.165, p = 0.028; putamen, estimate = 0.396, SE = 0.137, p = 0.016). For visualization purposes, we extracted subgroups who had GRS2 ≥ 84 percentile (mean + 1 SD) and GRS ≤ 16 percentile (mean – 1 SD). PD patients with GRS2 ≥ 84 percentile exhibited a slower decline in striatal DAT SBRs than those with GRS2 ≤ 16 percentile (Figure 2).
Conclusion: Our results suggest that genetic factors for PD risk may have heterogeneous effects on the striatal dopaminergic degeneration, and some factors may be associated with a slower decline of dopaminergic activity. Composition of PD-progression specific GRS may be useful in predicting disease progression in patients.
To cite this abstract in AMA style:
KJ. Pak, MJ. Lee. Effect of polygenic load on striatal dopaminergic deterioration in Parkinson’s disease [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/effect-of-polygenic-load-on-striatal-dopaminergic-deterioration-in-parkinsons-disease/. Accessed March 31, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/effect-of-polygenic-load-on-striatal-dopaminergic-deterioration-in-parkinsons-disease/