Session Information
Date: Wednesday, June 7, 2017
Session Title: Ataxia
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: To assess the acceptation level and ethical concerns for participating in early intervention approaches in Spinocerebellar Ataxia type 2 preclinical carriers and to evaluate the efficay and safety of a 8-week neurorehabilitation program.
Background: Spinocerebellar Ataxias are a neurodegenerative disorders characterized by degeneration of cerebellum, brainstem, spinal cord and peripheral nerves. In the past 20 years clinical trials have been carried out in patients with SCAs to slow down or stop disease progression and disability.
Methods: Fourty-five SCA2 preclinical carriers and 45 healthy controls were assessed by clinical neurological exams, ataxia rating scales and electrophysiological tests. All preclinical carriers were interviewed on their acceptation and expectations about early clinical trials and posteriorly 40 of them were included into an early intervention consisting on an 8-week neurorehabilitation program.
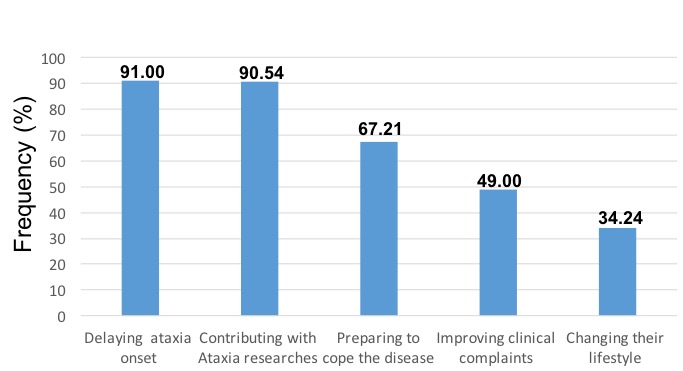
Results: The most common clinical manifestation in preclinical carriers were the muscle cramps, sensory abnormalities, hyperreflexia, dysautonomic disorders (nocturia, constipation and throat clearing), which were accompanied by early saccade slowing and increased antisaccadic error rates. The interview on the early interventions revealed a high acceptation level (98%) of these therapeutical approaches, mainly physical rehabilitation and placebo-controlled clinical trials of neuroprotective drugs. Most of preclinical carriers attest that would reveal their genetic status during the early interventions. The main reasons for participating in early interventions were: 1) to delay the ataxia onset (91%), 2) to contribute with the scientific researches (91%) and 3) to be prepared to cope the disease (67%) (Figure 1). Regarding the neurorehabilitation program, the majority of treated carriers exhibited a significant improvement of tandem gait, postural stability and limbs coordination, which difered from the untreated group, which kept relatively stable in this short period. The treatment was safe in all subjects minimizing the ethical dilemma associated to the therapy side effects.
Conclusions: Summarizing, the ethical concerns raised from the early interventions in prodromal SCA2 have led to new challenges to physicians, genetic counselors and researchers, which must to be addressed by further investigations.
References: Velázquez-Pérez L, Rodríguez-Labrada R, Cruz-Rivas EM, et al. Comprehensive study of early features in spinocerebellar ataxia 2: delineating the prodromal stage of the disease. Cerebellum 2014;13(5):568-579
Velázquez-Pérez L, Rodríguez-Labrada R, Canales-Ochoa N, et al. Progression of early features of spinocerebellar ataxia type 2 in individuals at risk: a longitudinal study. Lancet Neurol 2014;13(5):482-489.
To cite this abstract in AMA style:
L. Velázquez-Pérez, R. Rodríguez-Labrada, J. Rodríguez-Diaz, Y. Vazquez-Mojena, J. Medrano-Montero, A. Estupiñán-Rodríguez. Early diagnosis in Spinocerebellar Ataxias: Prospects for clinical alterations and ethical dilemmas during preclinical trials. [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/early-diagnosis-in-spinocerebellar-ataxias-prospects-for-clinical-alterations-and-ethical-dilemmas-during-preclinical-trials/. Accessed February 27, 2026.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/early-diagnosis-in-spinocerebellar-ataxias-prospects-for-clinical-alterations-and-ethical-dilemmas-during-preclinical-trials/