Category: Dystonia: Clinical Trials and Therapy
Objective: To evaluate the safety, feasibility, tolerability and effects of accelerated rTMS for treatment of cervical dystonia.
Background: In a subgroup of patients with cervical dystonia (CD), botulinum toxin (BoNT) injections do not last the full 12 weeks. Low-frequency repetitive transcranial magnetic stimulation (rTMS) therapy targeted at the dorsal premotor cortex (dPMC) is a promising adjunct therapy for limb dystonia, but has not yet been studied in CD.
Method: We enrolled 5 patients (4/5 female, average age 68.7 years, average dystonia duration 9.2 years) with CD and reporting BoNT benefit lasting ≤ 9 weeks in a double-blind, sham-controlled, crossover design study. At 9 weeks following BoNT, patients were randomized to 1-Hz rTMS (active or sham) over the dPMC for 30 minutes (1800 pulses) at 90% resting motor threshold, 4 sessions per day for 4 consecutive days. There was a 12 week washout period. Blinded video ratings of the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) motor subscores were obtained. We also recorded mood symptoms with Beck Depression Inventory (BDI), cognitive symptoms measured by the trail making test (TMT), and safety and tolerability measures via patient-reported outcomes. Two-way ANOVA was used for statistical analysis. Outcomes were collected before rTMS, immediately after, and 2 weeks after rTMS.
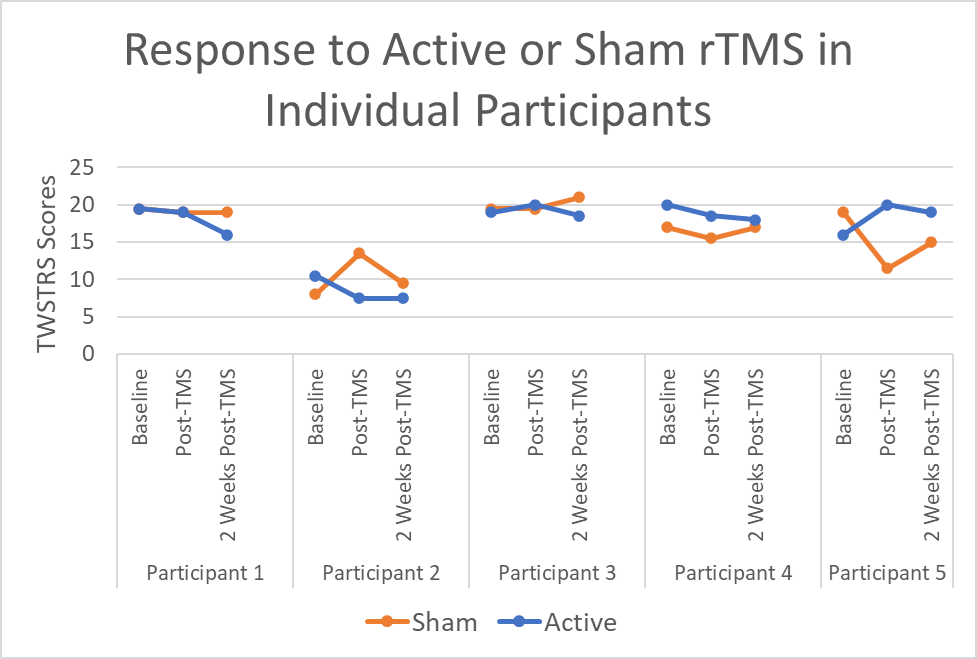
Results: Although there was a trend toward improvement in TWSTRS scores in the active group as compared to the sham group (Figure 1), this trend did not reach statistical significance compared to sham (p=0.777) or following rTMS (p=0.429). There was a significant difference in BDI scores between active and sham groups (p=0.0495). There were no statistical differences between TMT times for drawing A (p=0.2299) or drawing B (p=0.9222). All patients tolerated the stimulation well, with 3/5 patients reporting transient self-resolving headaches after active rTMS. In terms of blinding, 3/5 reported “could not tell” which coil was the sham, and 1/5 correctly identified the sham coil, suggesting effective blinding.
Conclusion: The use of novel, accelerated rTMS protocol in CD was found to be safe and well-tolerated. There were trends toward improvement following TMS, although future larger studies are needed to clarify these results. Combined use of rTMS and BoNT is promising and further work in larger samples is needed to understand the physiological and non-motor effects of this intervention.
To cite this abstract in AMA style:
J. Frey, J. Yu, J. Lobo Lopes, L. Fanty, I. Malaty, A. Ramirez-Zamora, M. Wajid, J. Cagle, C. de Hemptinne, A. Wagle Shukla. Dystonia Treatment With Injections Supplemented by TMS: the D-TWIST Study [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/dystonia-treatment-with-injections-supplemented-by-tms-the-d-twist-study/. Accessed February 27, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/dystonia-treatment-with-injections-supplemented-by-tms-the-d-twist-study/