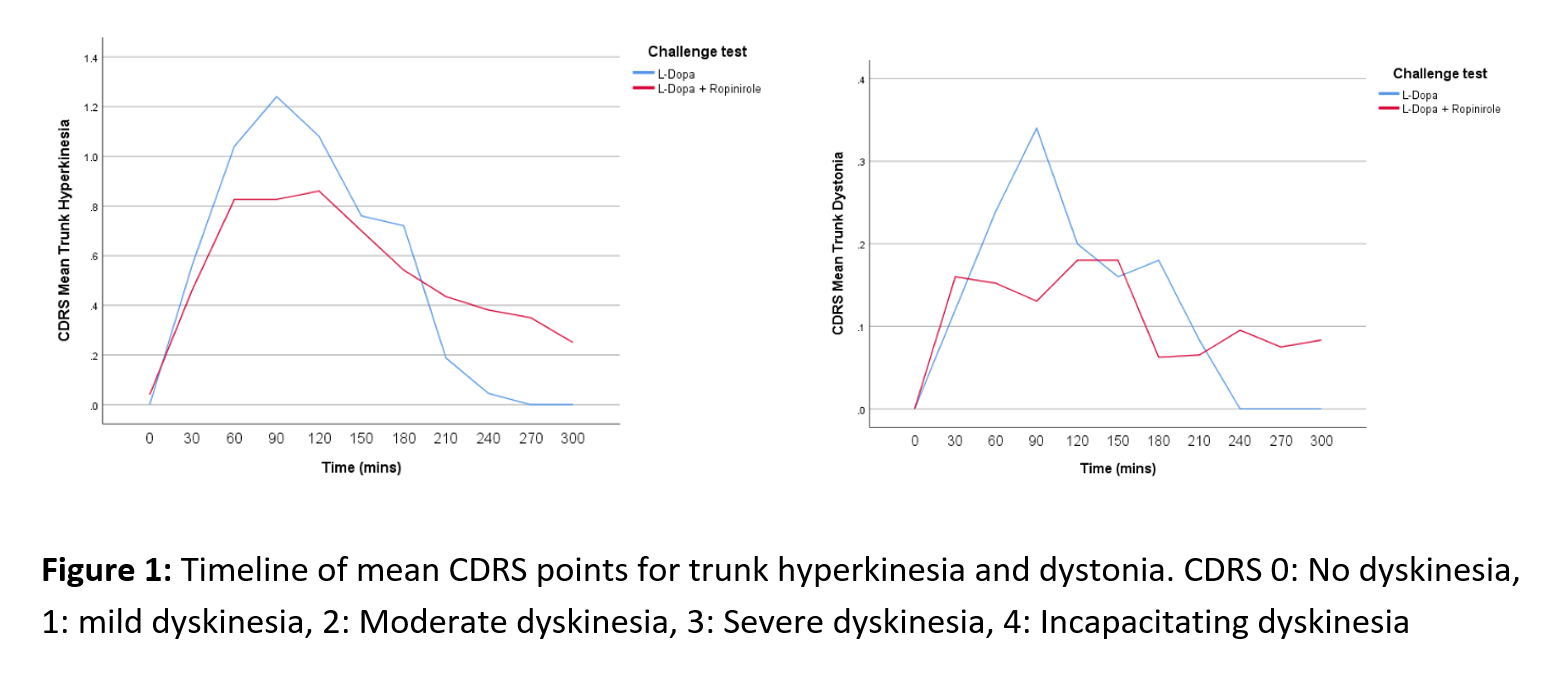
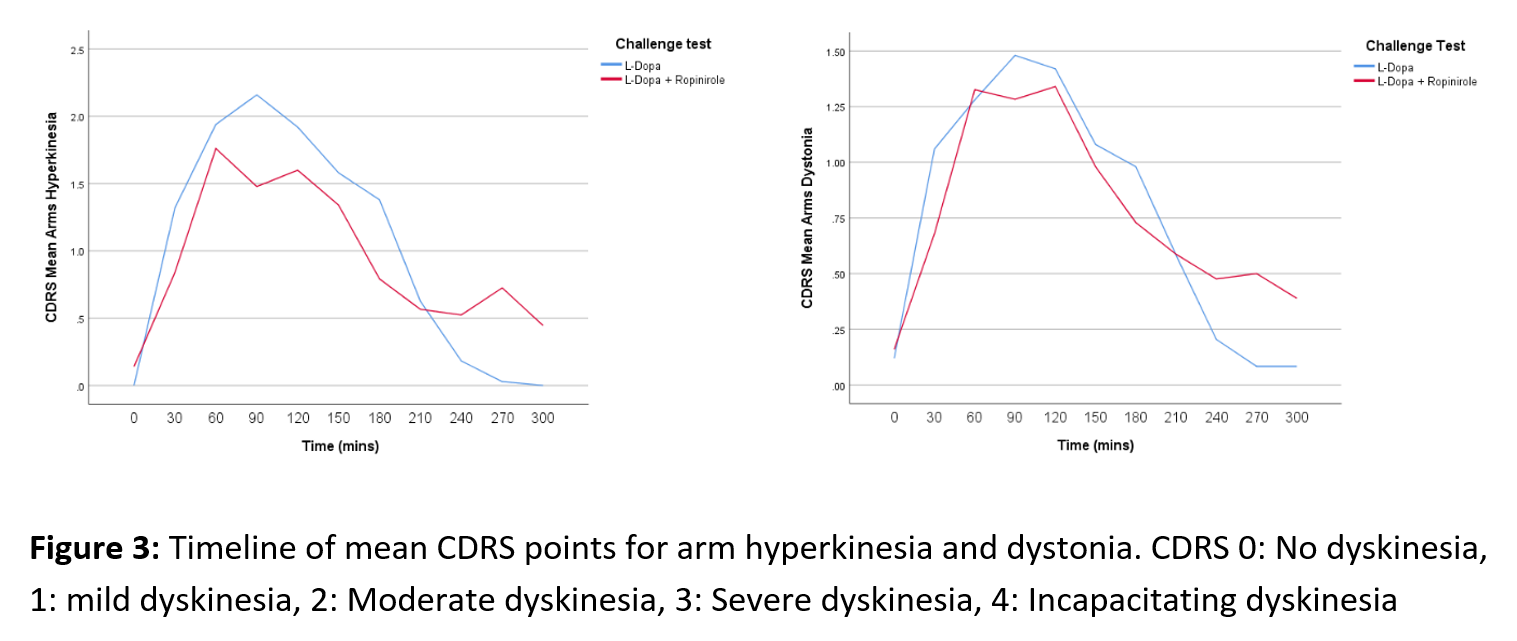
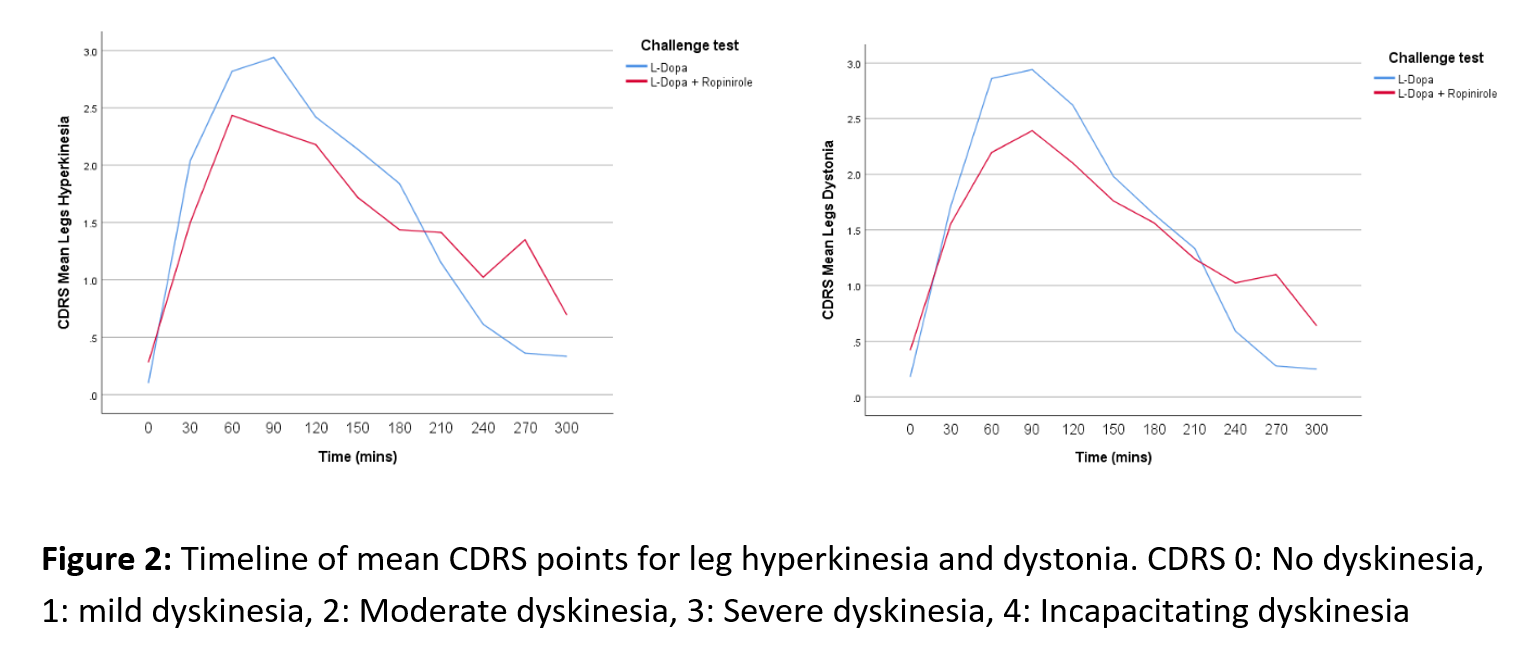
Objective: To investigate possible differences in the profiles of abnormal involuntary movements (AIMs) in patients with Parkinson’s disease (PD) after dose challenge with L-dopa alone or L-dopa combined with ropinirole.
Background: Dyskinesias are common in PD patients with a long duration of disease. The term encompasses different types of AIMs having hyperkinetic or dystonic character. The underlying pathophysiology involves neuronal circuits that are differentially regulated by the two main classes of dopamine receptors, D1R and D2R. While L-dopa stimulates both receptor classes, ropinirole has selective affinity for the D2R and has, in addition, a longer half-life than L-dopa. Recent animal studies indicate that selective D2R stimulation predominantly mediates dystonic AIMs components.
Method: 25 patients with PD and a history of dyskinesias were included. Patients received challenge tests on two occasions using either L-dopa alone (150% of usual morning dose) or a combination of L-dopa and ropinirole with equal levodopa-equivalent potency. The challenge tests were performed after pausing all antiparkinsonian agents for one day, with amantadine being paused 1 week prior to the first challenge test. All patients received both challenge tests in a random order and within 2 weeks. AIMs were rated prior and every 30 minutes after drug dosing using the Clinical Dyskinesia Rating Scale (CDRS), which yields separate ratings of hyperkinesia and dystonia in different body parts.
Results: An initial statistical analysis suggests the dyskinesia time curves differ between treatments as the L-dopa-ropinirole combination generally resulted in lower peak severity, but longer duration of the AIMs compared with L-dopa-only. At the peak of the AIMs curve (60-120 min), L-dopa induced significantly (p < 0.05) higher levels of hyperkinesias in the trunk and the legs, and higher leg and trunk dystonia scores (Fig. 1-2). In the end phase of the AIMs curve (240-270 min), there was a consistent trend for more severe AIMs after challenge with levodopa-ropinirole, which reached statistical significance for the arm dystonia item (Fig. 3).
Conclusion: To our knowledge, this is the first study to examine the impact of D2R agonist coadministration on the phenomenology of L-DOPA-induced dyskinesias. The data so far indicate that D2R stimulation results in more severe end phase AIMs, particularly evident on the arm dystonia item.
References: [1] Tran TN, Vo TNN, Frei K, Truong DD. Levodopa-induced dyskinesia: clinical features, incidence, and risk factors. Journal of Neural Transmission. 2018;125(8):1109-17.
[2] Cenci MA, Jorntell H, Petersson P. On the neuronal circuitry mediating LDOPA-induced dyskinesia. J Neural Transm (Vienna). 2018;125(8):1157-69.
[3] Hagell P, Widner H. Clinical rating of dyskinesias in Parkinson’s disease: use and reliability of a new rating scale. Mov Disord. 1999;14(3):448-55.
[4] Andreoli L, Abbaszadeh M, Cao X, Cenci MA. Distinct patterns of dyskinetic and dystonic features following D1 or D2 receptor stimulation in a mouse model of parkinsonism. Neurobiol Dis. 2021 Sep;157:105429.
An earlier abstract of the same study (with the first 12 patients) was published at the MDS Virtual Congress 2020.
S. Grigoriou, S. Christiansson, P. Odin, M.A Cenci. Impact of dopamine 2/3 receptor agonists on the phenomenology of L-DOPA-induced dyskinesia in patients with Parkinson’s disease [abstract]. Mov Disord. 2020; 35 (suppl 1).
To cite this abstract in AMA style:
S. Grigoriou, P. Odin, E. Espa, A. Cenci. Dyskinesia profiles after dose challenges with L-dopa vs L-dopa plus ropinirole: a pilot study [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/dyskinesia-profiles-after-dose-challenges-with-l-dopa-vs-l-dopa-plus-ropinirole-a-pilot-study/. Accessed April 18, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/dyskinesia-profiles-after-dose-challenges-with-l-dopa-vs-l-dopa-plus-ropinirole-a-pilot-study/