Session Information
Date: Thursday, June 8, 2017
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective:
The TOLEDO Study (NCT02006121) is a prospective, randomized, multicenter, double-blind, Phase III trial undertaken to investigate the efficacy of apomorphine subcutaneous infusion (APO) compared with placebo in Parkinson’s disease (PD) patients with motor fluctuations not well controlled on optimized conventional treatment.
Background:
APO is a well-established therapy for the management of PD patients with severe motor fluctuations whose symptoms are poorly controlled by conventional therapy. Open-label clinical studies have shown APO to be effective in reducing OFF time, dyskinesias and oral levodopa dose, but confirmatory evidence from randomized, blinded studies has not been available until now.
Methods:
Patients from 23 centers in 7 countries were randomized to receive APO during their waking time (16±2 hours) at up to 8 mg/hour, or placebo saline infusion administered by the same pump system, for 12 weeks. Based on individual efficacy and tolerability, the hourly flow rate of the study drug and doses of concomitant antiparkinsonian medication were adjusted during the first 4 weeks. The primary endpoint was the absolute change in OFF time from baseline (BL) to Week 12 based on patient home diaries.
Results:
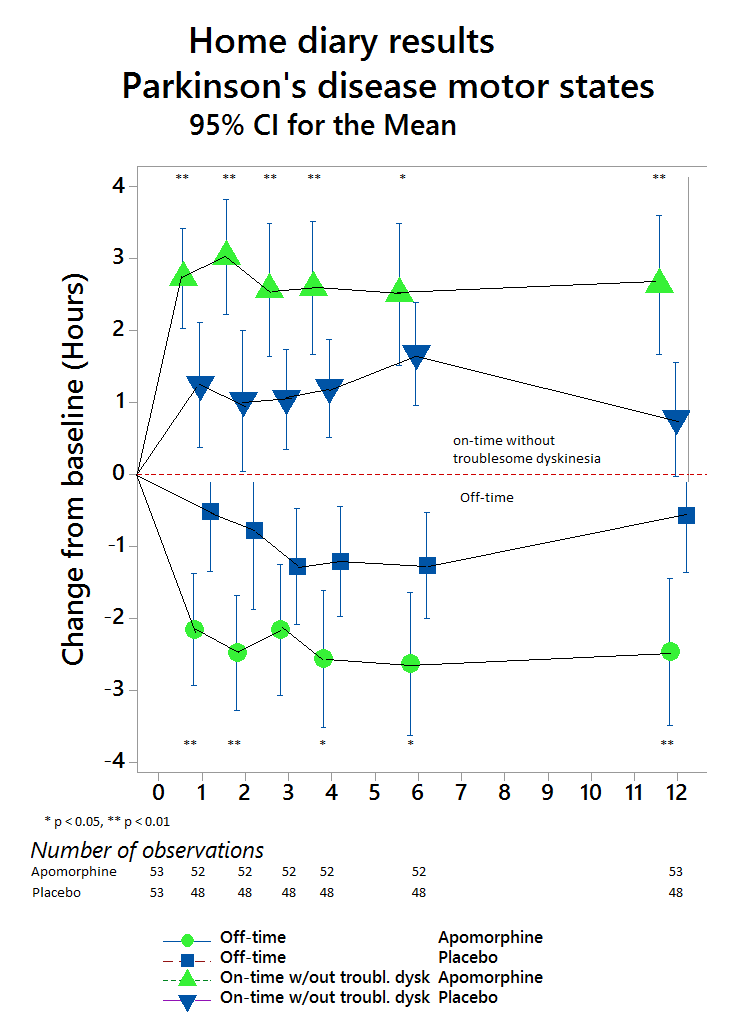
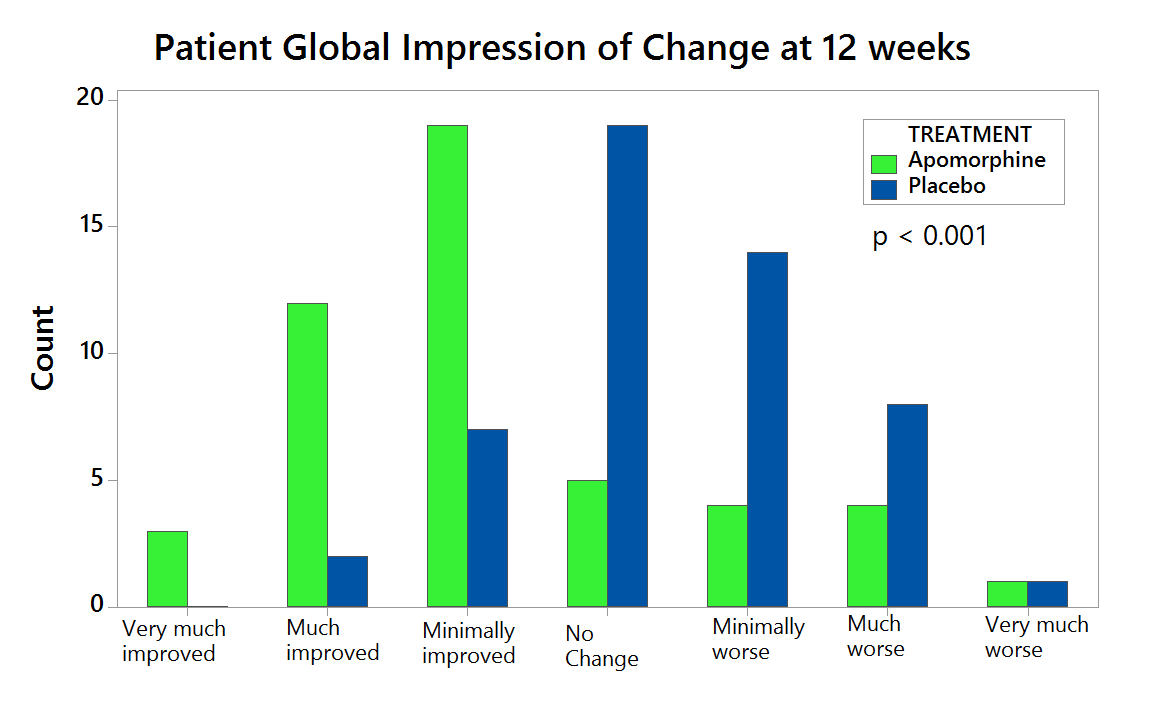
Compared with placebo (n=53), APO (n=53) provided significantly greater reduction in OFF time between BL and Week 12 (-0.58 hours versus -2.47 hours, respectively), a difference between treatment groups of -1.89 hours (95% CI: -3.16, -0.62; p=0.0025). (Figure 1). APO also led to a significantly greater increase in ON time without troublesome dyskinesia. The beneficial effects of APO were clinically meaningful for patients as reflected in better scores for Patient Global Impression of Change versus placebo (Figure 2). APO was generally well tolerated and no unexpected safety signals were reported.
Conclusions:
These results provide the first Level 1 evidence from a multicenter, double-blind, randomized, placebo-controlled trial to confirm that APO significantly and consistently reduces OFF time in this patient group while significantly increasing ON time without troublesome dyskinesia.
To cite this abstract in AMA style:
R. Katzenschlager, W. Poewe, O. Rascol, C. Trenkwalder, G. Deuschl, R. Chaudhuri, T. Henriksen, T. van Laar, K. Spivey, S. Vel, A. Lees. Double-blind, randomized, placebo-controlled, Phase III study (TOLEDO) to evaluate the efficacy of apomorphine subcutaneous infusion in reducing OFF time in Parkinson’s disease patients with motor fluctuations not well controlled on optimized conventional treatment [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/double-blind-randomized-placebo-controlled-phase-iii-study-toledo-to-evaluate-the-efficacy-of-apomorphine-subcutaneous-infusion-in-reducing-off-time-in-parkinsons-disease-patients-with-m/. Accessed December 28, 2025.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/double-blind-randomized-placebo-controlled-phase-iii-study-toledo-to-evaluate-the-efficacy-of-apomorphine-subcutaneous-infusion-in-reducing-off-time-in-parkinsons-disease-patients-with-m/