Category: Dystonia: Clinical Trials and Therapy
Objective: To perform a systematic review to identify relevant literature on pain outcomes after injecting BTX with a microneedle for various facial conditions and to provide an evidence-based recommendation for microneedle use.
Background: Botulinum toxin (BTX) has revolutionised the treatment of patients who have facial conditions due to neurological, dermatological, and ophthalmological disorders. As the efficacy of a single BTX injection lasts between 3-6 months, repeated, potentially painful, injections are required for patients to achieve sustained benefits. To try to reduce injection discomfort, several studies using a microneedle, a needle smaller than a standard 30-gauge (G) needle, have been conducted.
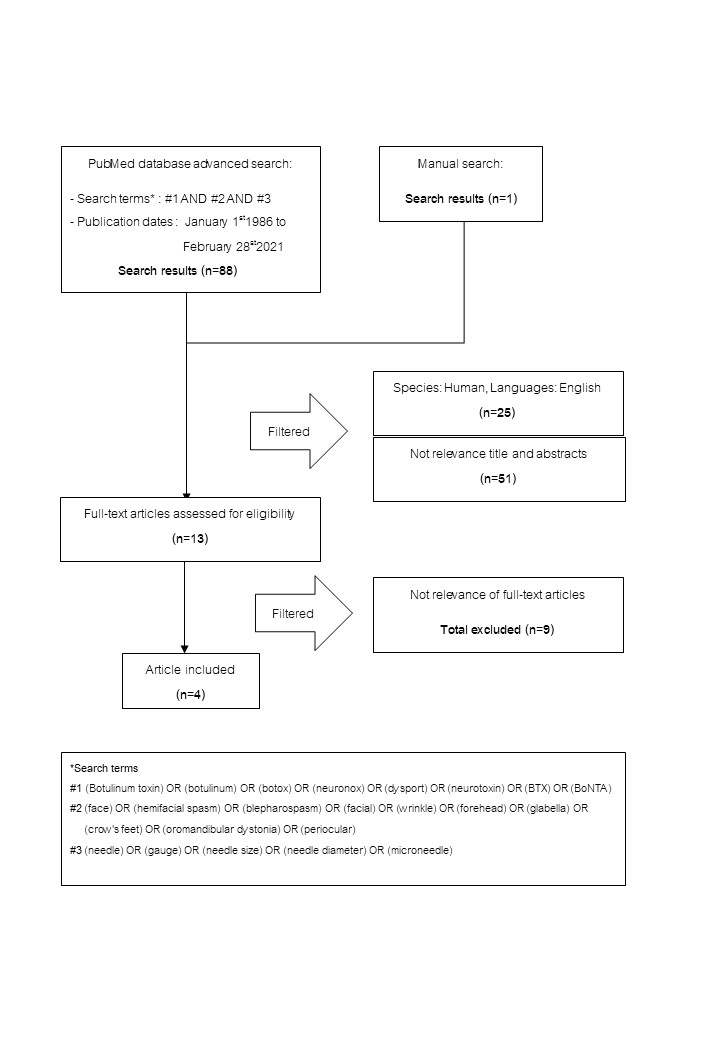
Method: Systematic reviews were conducted by searching PsycINFO, Ovid MEDLINE, EMBASE, Web of Science and Cochrane for articles published between January 1986 to February 2021 according to the PRISMA guidelines. Articles were classified according to the American Academy of Neurology (AAN) 4-tiered evidence-rating scheme. Recommendations were based on the evidence found.
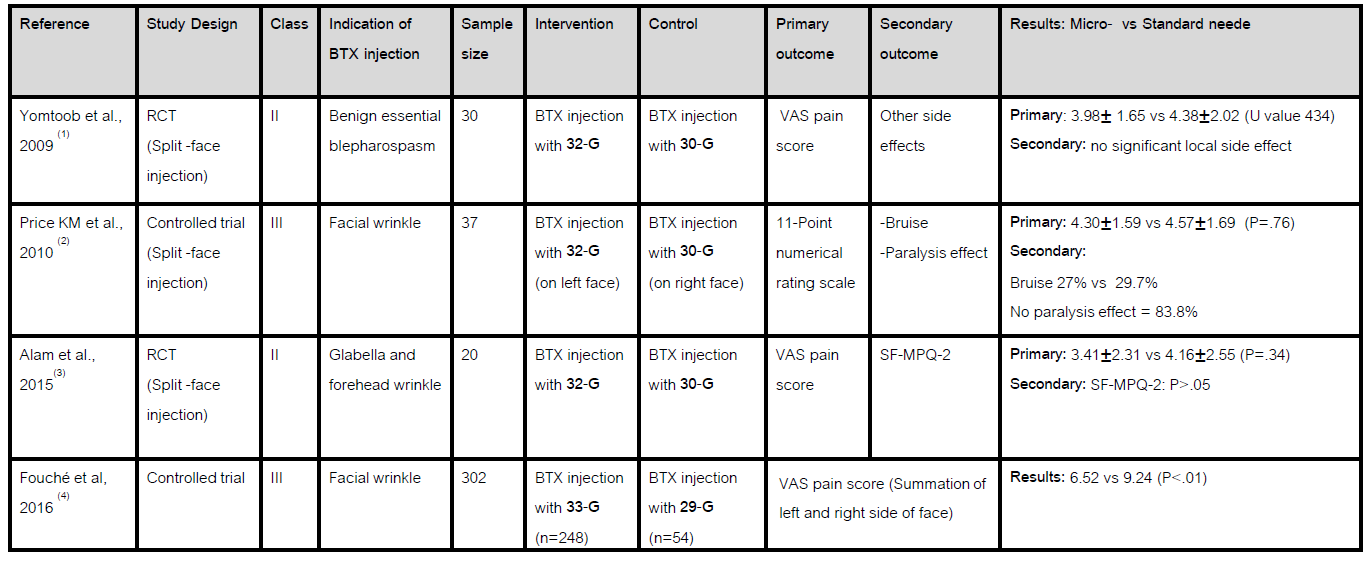
Results: Of 88 abstracts screened, 4 articles fulfilled the selection criteria that assessed pain outcomes after injecting BTX with a microneedle for various facial conditions. There was no difference in pain outcomes from 3 articles (two class II and one class III) which compared BTX injection with a 32-G needle and a 30-G needle. However, another one class III article, which compared pain outcomes after BTX injection with a 33-G needle and a 29-G needle, found facial injection with a 33-G needle was less painful than injection with a 29-G needle.
Conclusion: Based on the AAN level of evidence, using a 32-G needle for facial area BTX injection is probably ineffective to reduce pain compared to a 30-G needle (Level B). However, there is insufficient evidence to support use of a 33-G needle as less painful than a 29-G needle (Level U). More studies with standardised pain outcome measures are needed to determine if the use of smaller needles (29-G or 30-G) is associated with lesser pain after BTX injections.
References: 1. Yomtoob DE, Dewan MA, Lee MS, Harrison AR. Comparison of pain scores with 30-gauge and 32-gauge needles for periocular botulinum toxin type a injections. Ophthalmic Plastic & Reconstructive Surgery. 2009;25(5):376-7. 2. Price KM, Williams ZY, Woodward JA. Needle preference in patients receiving cosmetic botulinum toxin type A. Dermatologic surgery. 2010;36(1):109-12. 3. Alam M, Geisler A, Sadhwani D, Goyal A, Poon E, Nodzenski M, et al. Effect of needle size on pain perception in patients treated with botulinum toxin type A injections: a randomized clinical trial. JAMA dermatology. 2015;151(11):1194-9. 4. Fouché J, Van loghem J, Thuis J, de Heer L, Oijen M. Left/Right Pain Asymmetry With Injectable Cosmetic Treatments for the Face. Aesthetic surgery journal. 2017;37.
To cite this abstract in AMA style:
S. Maytharakcheep. Does Microneedle Use for Facial Injection of Botulinum Toxin Reduce Pain? A Systematic Literature Review [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/does-microneedle-use-for-facial-injection-of-botulinum-toxin-reduce-pain-a-systematic-literature-review/. Accessed February 6, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/does-microneedle-use-for-facial-injection-of-botulinum-toxin-reduce-pain-a-systematic-literature-review/