Category: Parkinson's Disease: Neuroimaging
Objective: To verify if freezing of gait (FOG) in Parkinson’s disease (PD) and gait automaticity share a similar neural substrate.
Background: FOG in PD may be due to a progressive loss of gait automaticity.1 People with PD and FOG (freezers) usually show higher dual-task cost (DTC) on stride length and gait speed, which may be linked to further impaired gait automaticity compared to people with PD without FOG.2, 3 Impaired gait automaticity requires more cognitive (e.g., increased dorsolateral prefrontal cortex [DLPFC] activation) and less locomotor (e.g., decreased cerebellar and mesencephalic locomotor region [CLR and MLR, respectively] activation) resources.4 Thus, if FOG and loss of gait automaticity in PD are linked, this could suggest that they share similar neural substrates; however, that is still unknown.
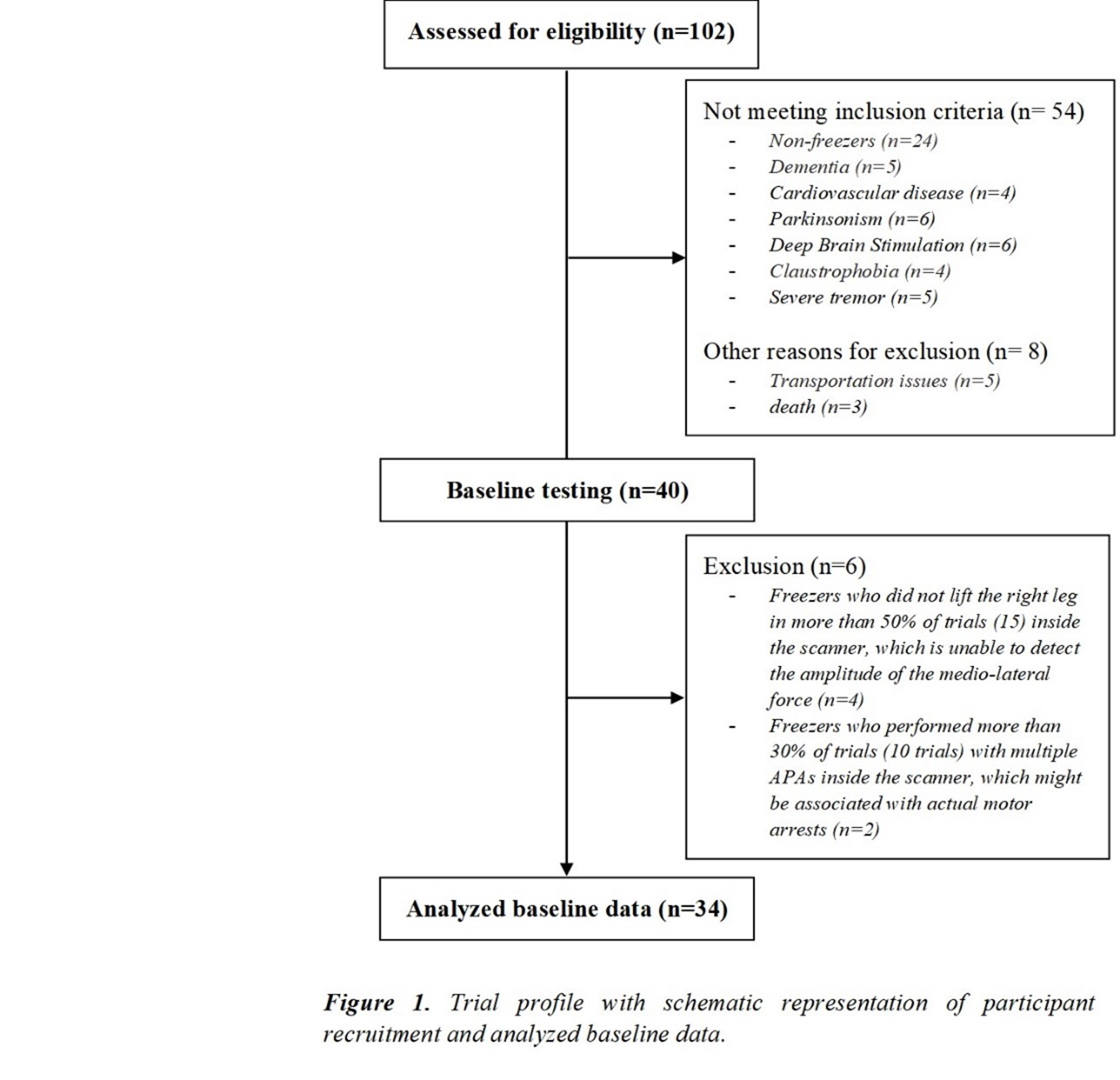
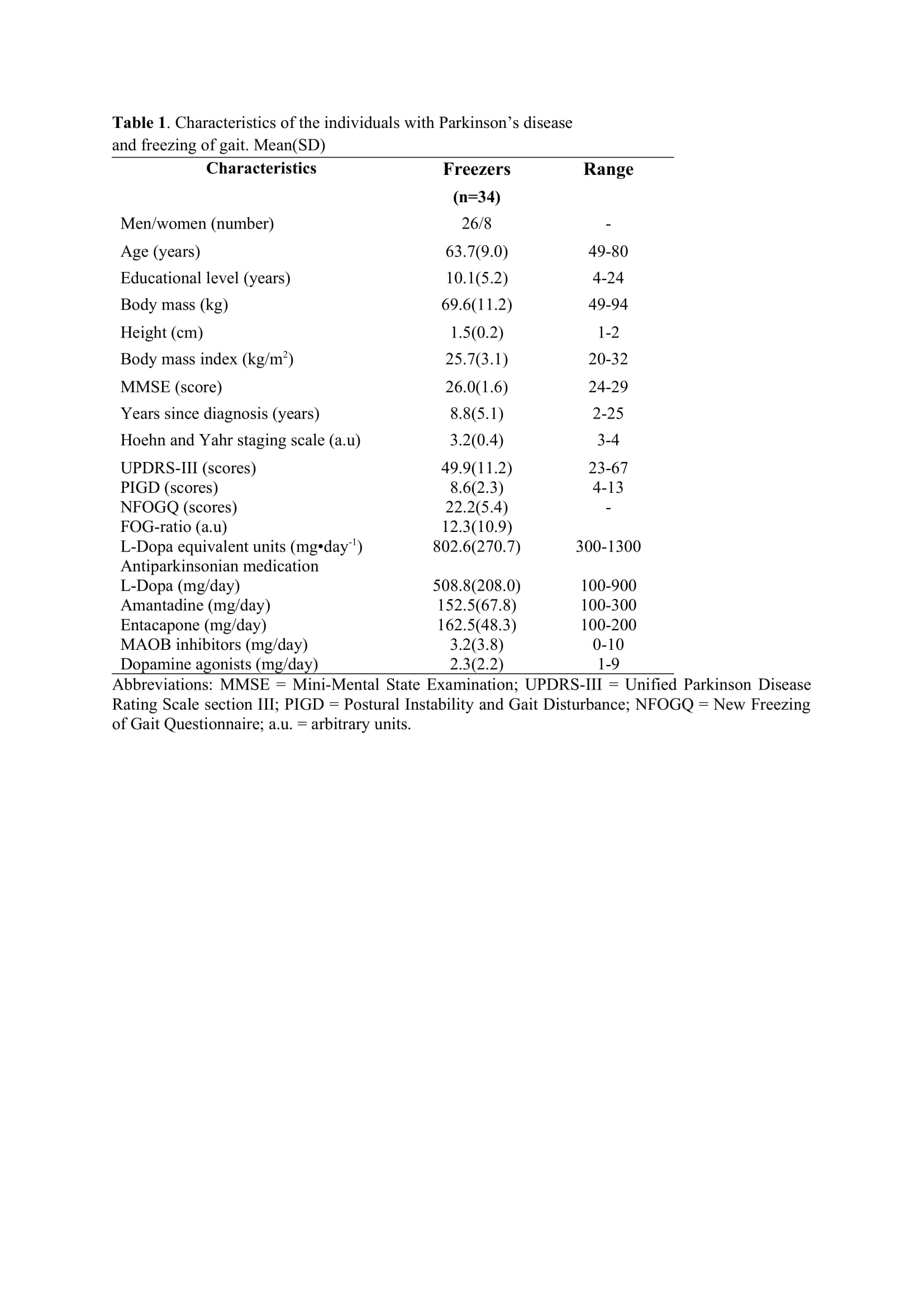
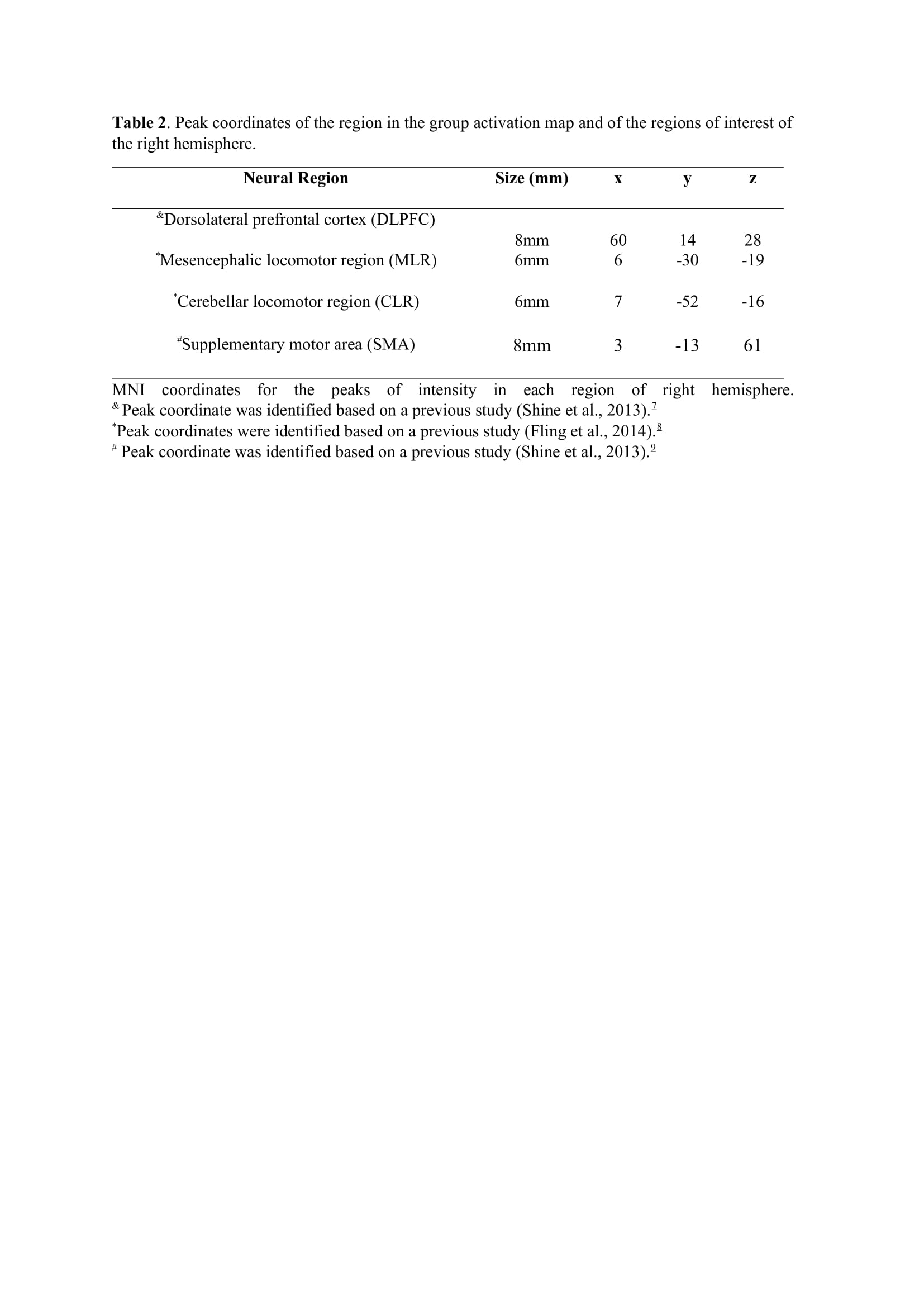
Method: Thirty-four freezers (Figure 1) were assessed in the ON-medication state (Table 1). We assessed the following: 1) FOG-ratio during turning test; 2) gait automaticity (DTC on stride length [m] and gait speed [m/sec] under single and dual-task conditions, 3 trials); 3) executive function (inhibitory control [Stroop-III test score] and attentional set-shifting [TMTB-A score]); and 4) beta of BOLD signal change (event-related fMRI protocol during step initiation)5, 6 from the regions of interest (DLPFC, MLR, CLR, and supplementary motor area) related to executive control and gait automaticity (Table 2).
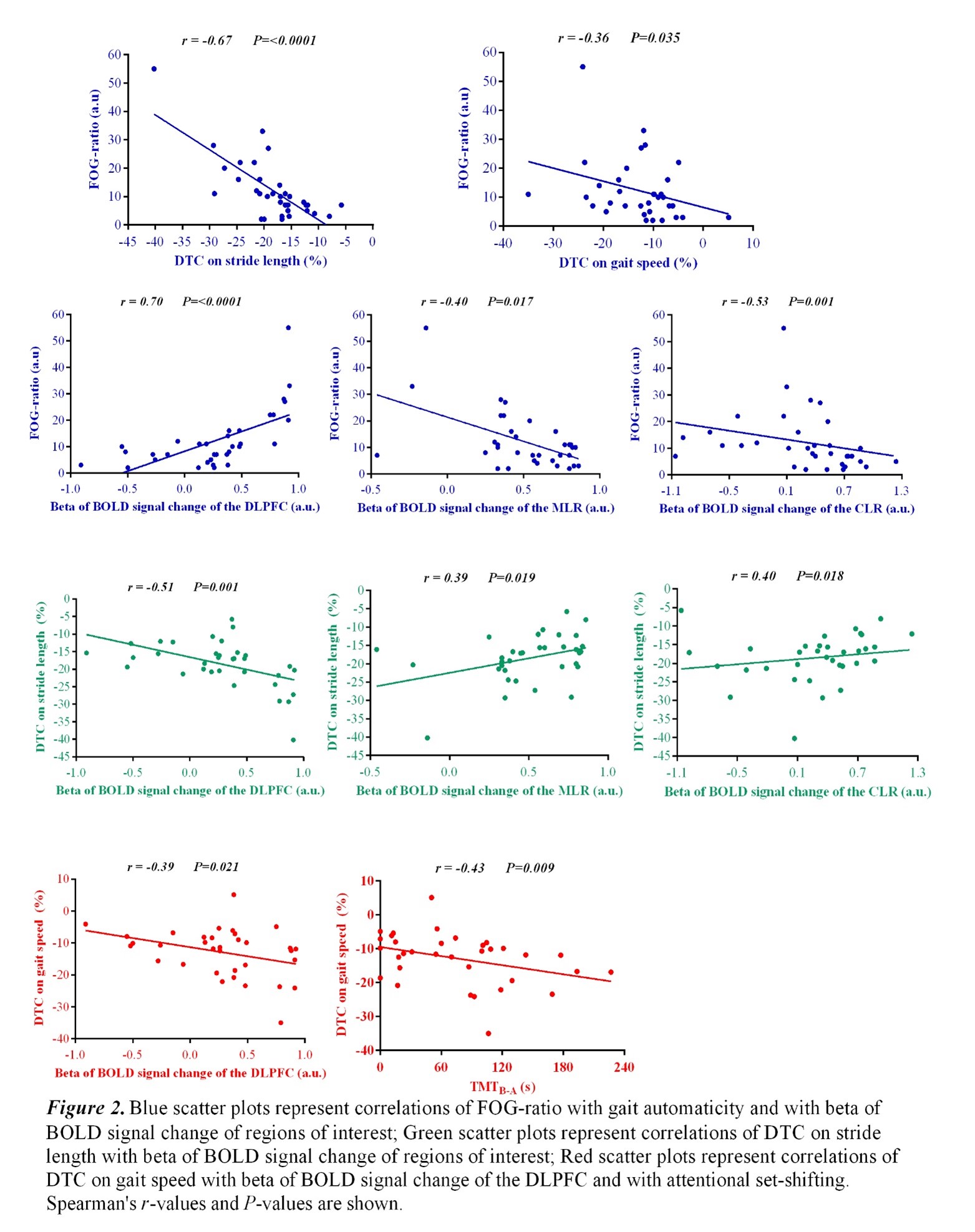
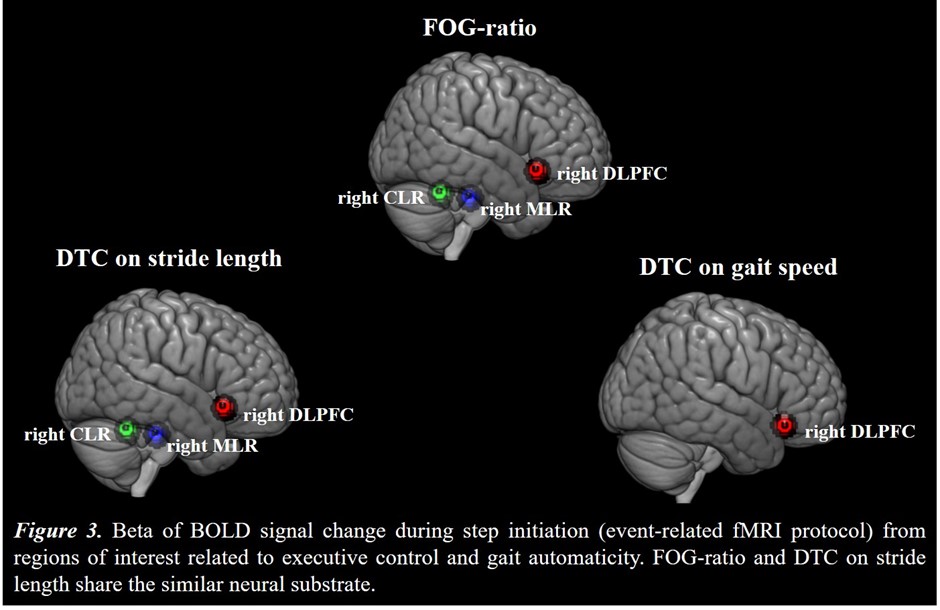
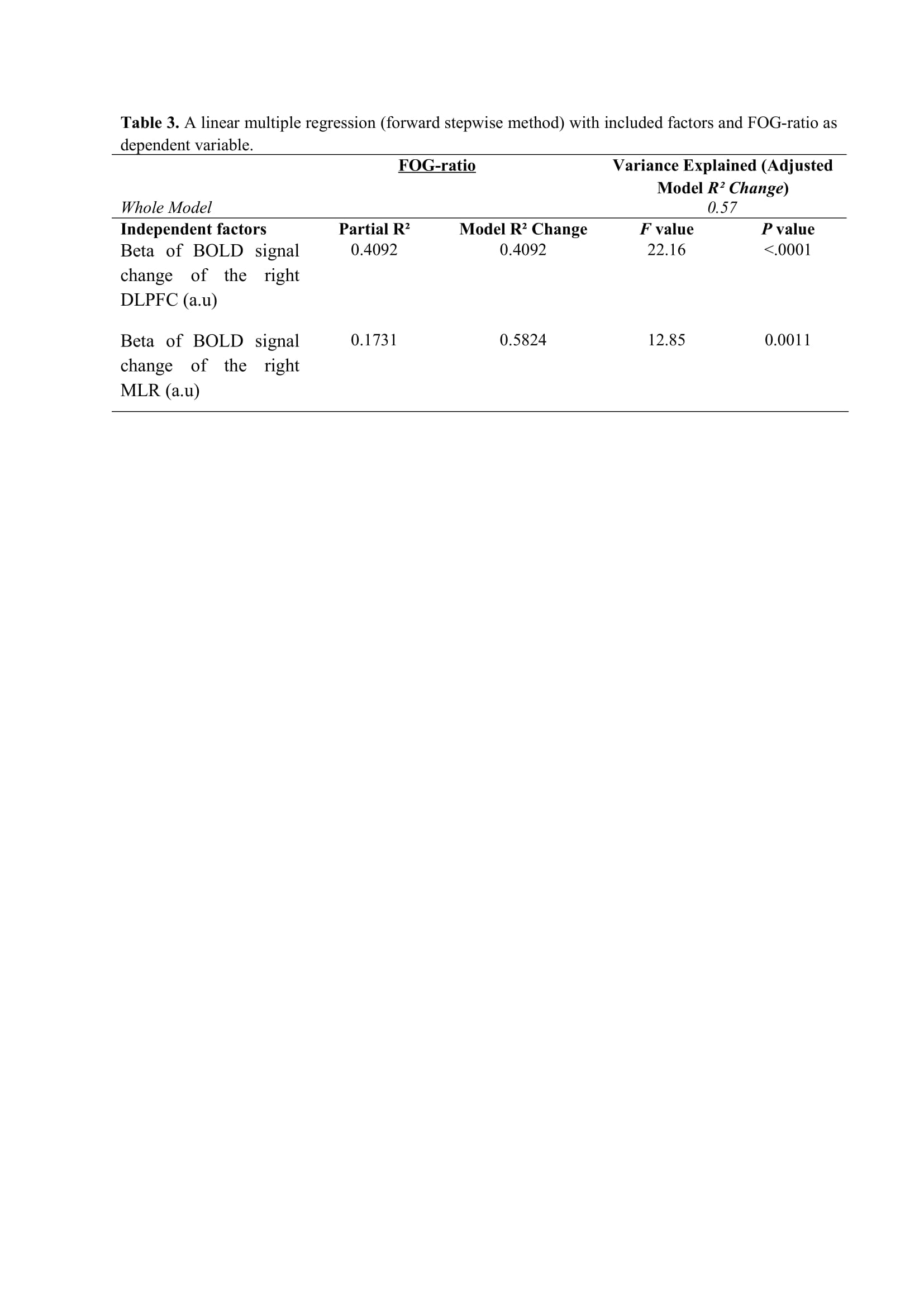
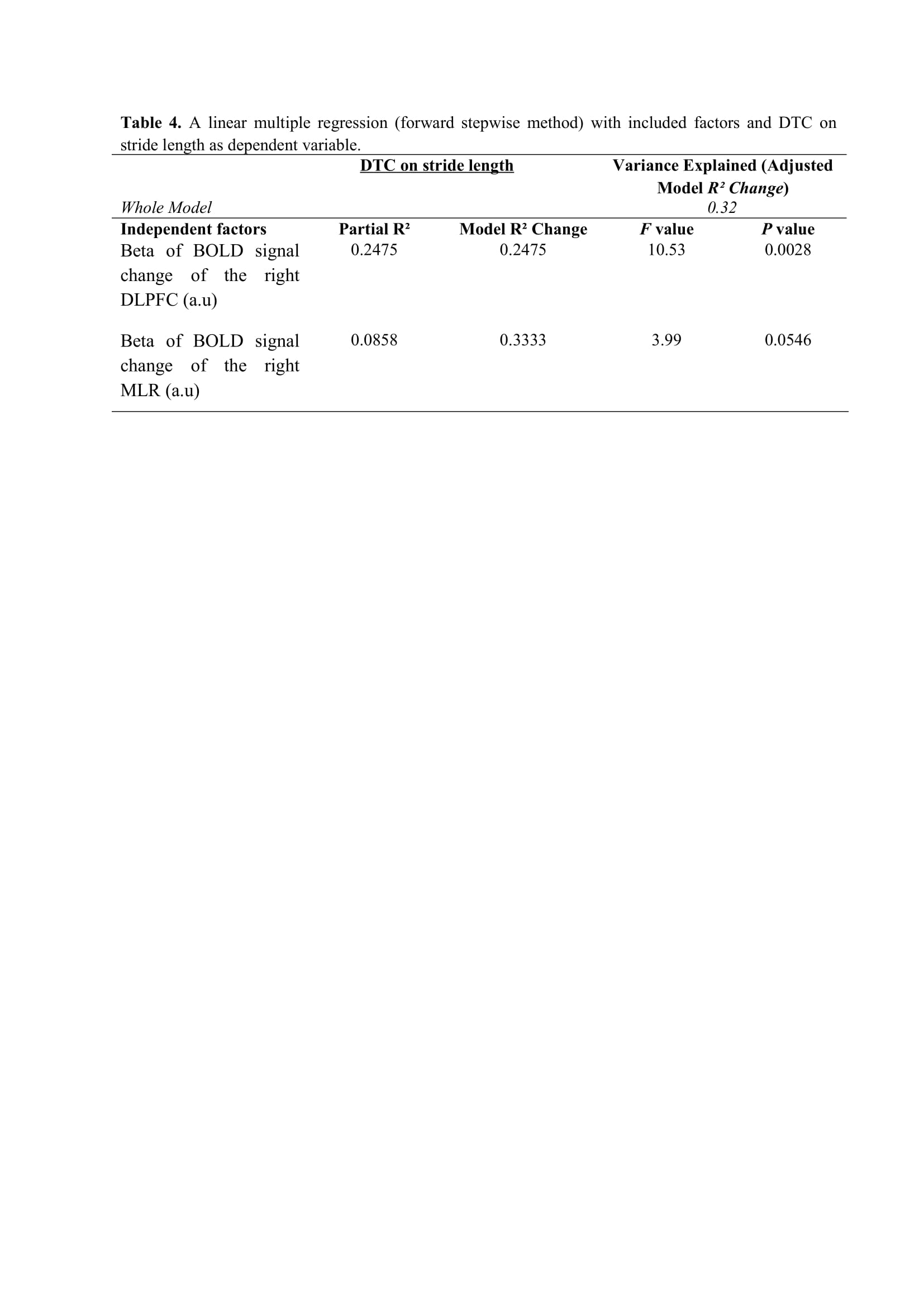
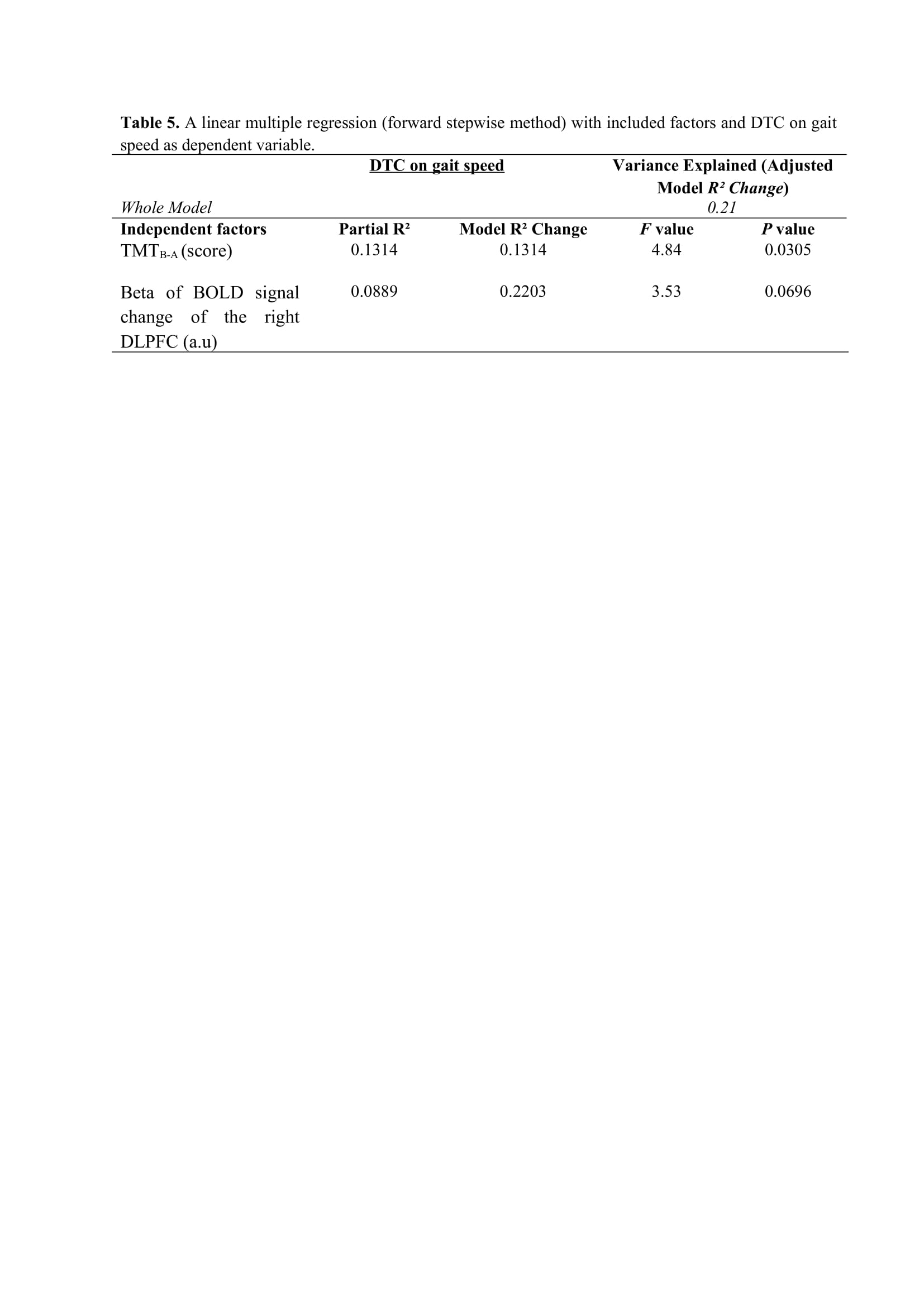
Results: Figure 2 shows that FOG-ratio was negatively associated with DTC on stride length and gait speed (P<0.05), BOLD signal of the right DLPFC was positively and negatively associated with FOG-ratio and DTC on stride length and gait speed, respectively (P<0.05), and BOLD signals of the right MLR and CLR were negatively and positively associated with FOG-ratio and DTC on stride length, respectively (P<0.05) (see Figure 3). Linear multiple regression approaches showed that BOLD signal of the right DLPFC and right MLR explained 57% (P<0.01) of the FOG-ratio variance (Table 3) and 32% (P≤0.05) of the DTC on stride length variance (Table 4), while attentional set-shifting explained 13% (P=0.03) of the DTC on gait speed variance (Table 5).
Conclusion: FOG severity and poor automaticity in stride length may require similar cognitive and locomotor recourses during step initiation in freezers, as DTC on stride length is the strongly gait parameter related to FOG-ratio.
References: 1. Vandenbossche J, Deroost N, Soetens E, et al. Freezing of gait in Parkinson’s disease: disturbances in automaticity and control. Frontiers in human neuroscience 2012;6:356. 2. de Souza Fortaleza AC, Mancini M, Carlson-Kuhta P, et al. Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait & posture 2017;56:76-81. 3. Vieira-Yano B, Martini DN, Horak FB, et al. The Adapted Resistance Training with Instability Randomized Controlled Trial for Gait Automaticity. Movement disorders : official journal of the Movement Disorder Society 2021;36(1):152-163. 4. Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain : a journal of neurology 2005;128(Pt 10):2250-2259. 5. de Lima-Pardini AC, de Azevedo Neto RM, Coelho DB, et al. An fMRI-compatible force measurement system for the evaluation of the neural correlates of step initiation. Scientific reports 2017;7:43088. 6. Silva-Batista C, de Lima-Pardini AC, Nucci MP, et al. A Randomized, Controlled Trial of Exercise for Parkinsonian Individuals With Freezing of Gait. Movement disorders : official journal of the Movement Disorder Society 2020;35(9):1607-1617. 7. Shine JM, Matar E, Ward PB, et al. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain : a journal of neurology 2013;136(Pt 4):1204-1215. 8. Fling BW, Cohen RG, Mancini M, et al. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PloS one 2014;9(6):e100291. 9. Shine JM, Matar E, Ward PB, et al. Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain : a journal of neurology 2013;136(Pt 12):3671-3681.
To cite this abstract in AMA style:
F. de Almeida, C. Ugrinowitsch, M. Nucci, D. Coelho, A. Moreira-Neto, E. Barbosa, L. Teixeira, E. Amaro JR., F. Horak, M. Mancini, C. Silva-Batista. DO FREEZING OF GAIT AND GAIT AUTOMATICITY IN PARKINSON’S DISEASE SHARE THE SIMILAR NEURAL SUBSTRATE? [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/do-freezing-of-gait-and-gait-automaticity-in-parkinsons-disease-share-the-similar-neural-substrate/. Accessed December 30, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/do-freezing-of-gait-and-gait-automaticity-in-parkinsons-disease-share-the-similar-neural-substrate/