Category: Parkinson's Disease: Neuroimaging
Objective: We aimed to identify differential patterns of aberrant tremor network microstructure using DSI among the following groups: (1) ET, (2) PD, and (3) healthy controls.
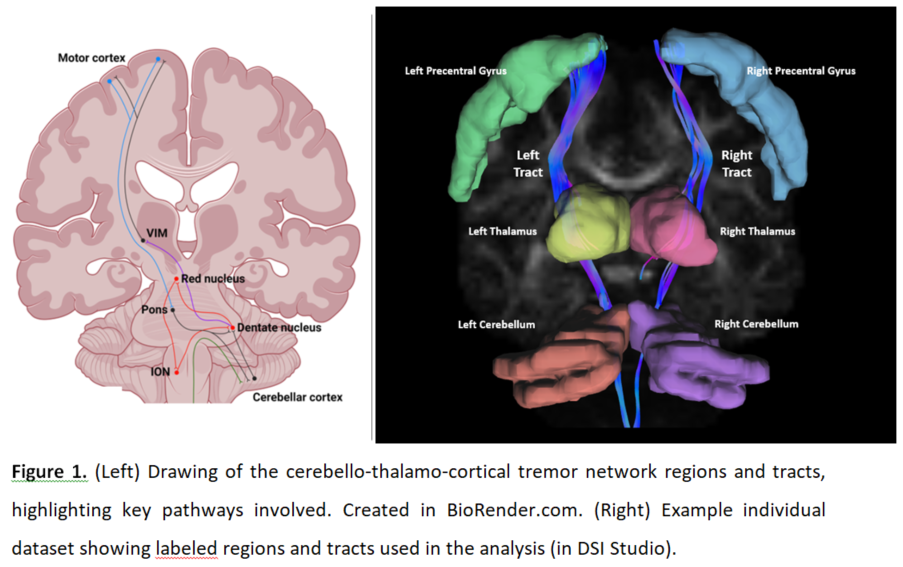
Background: Differentiation of essential tremor (ET) and Parkinson’s disease (PD) can be clinically challenging. ET and PD have contrasting neuropathological profiles but both involve a “tremor-network” in the brain (Figure 1L) [1]. Diffusion spectrum MRI (DSI) allows more detailed assessment of microstructure than conventional MRI [2]. We used DSI to identify differential patterns of aberrant tremor-network microstructure among ET, PD, and healthy controls.
Method: Subjects underwent clinical motor assessment (TETRAS, UPDRS), real-time motion-sensor testing [3], and DSI. We modelled the tremor-network using DSI Studio [4] (Figure 1R). We compared the groups on DSI metrics in each part of the tremor-network while controlling the false-discovery-rate. We used principal-components-analysis (PCA) to extract a pattern of disease-specific tremor-network microstructure and tested how strongly it was expressed in each group, and its correlation with tremor-severity.
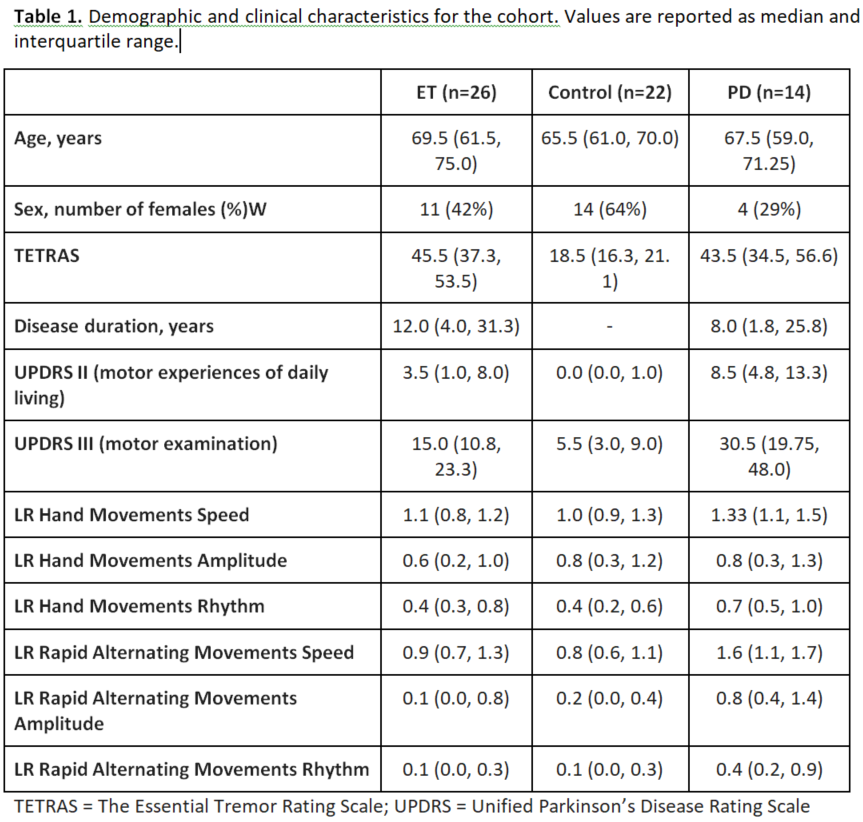
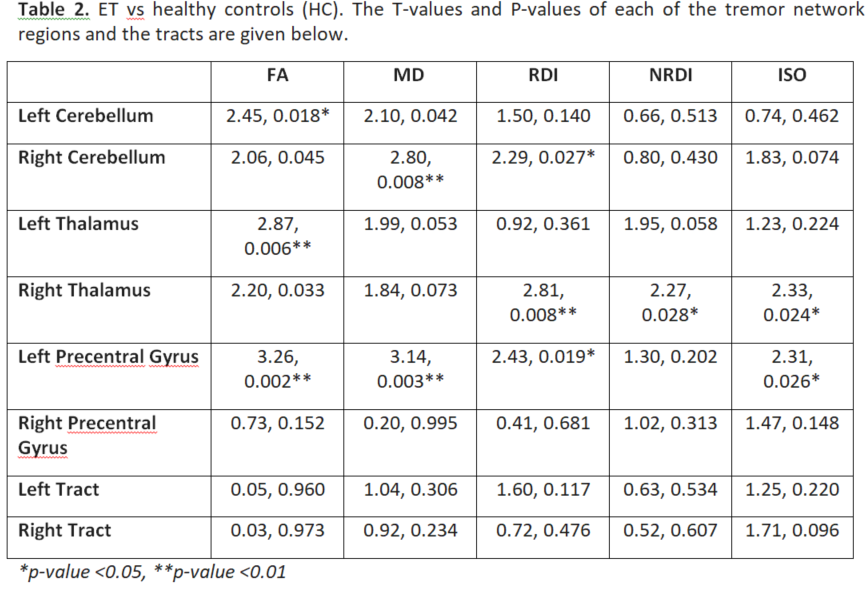
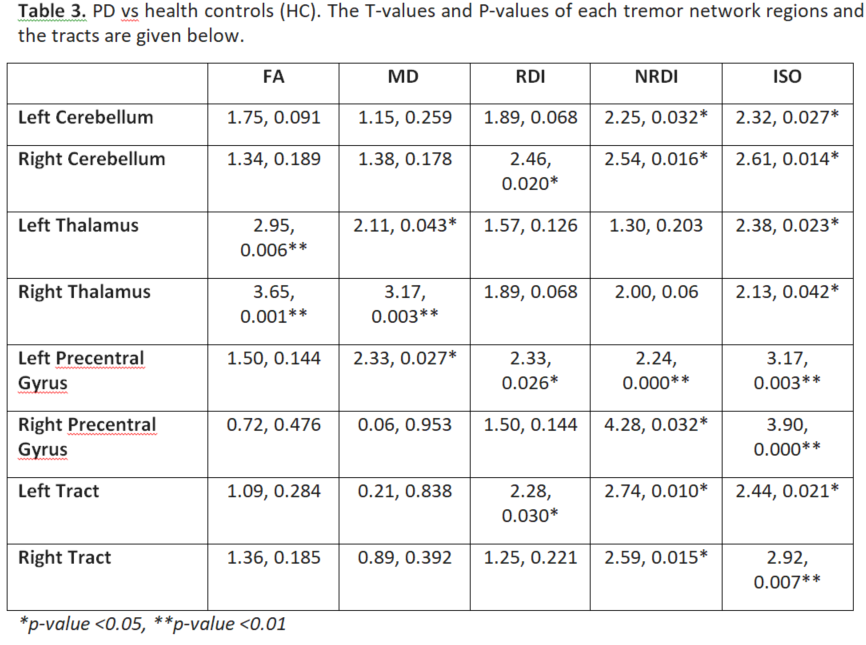
Results: Table 1 shows the sample characteristics (n=62). Both PD and ET groups had significant differences from controls in DSI characteristics of the tremor-network, which only minimally overlapped spatially (Tables 2 and 3).
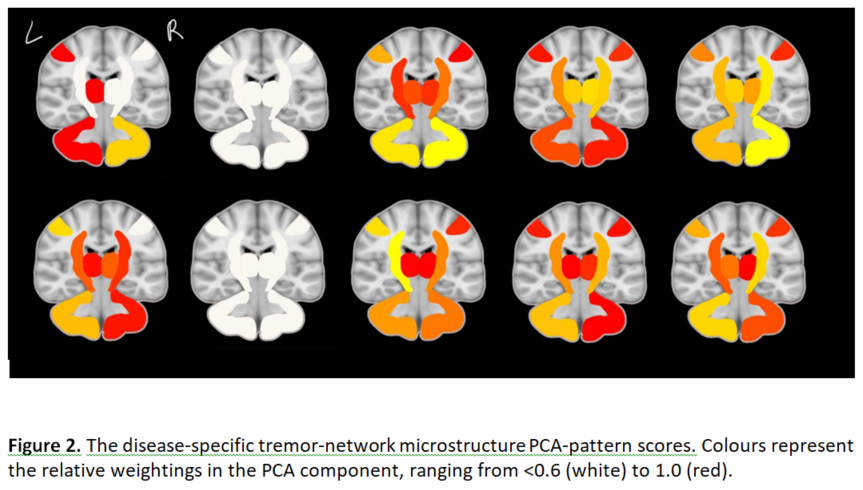
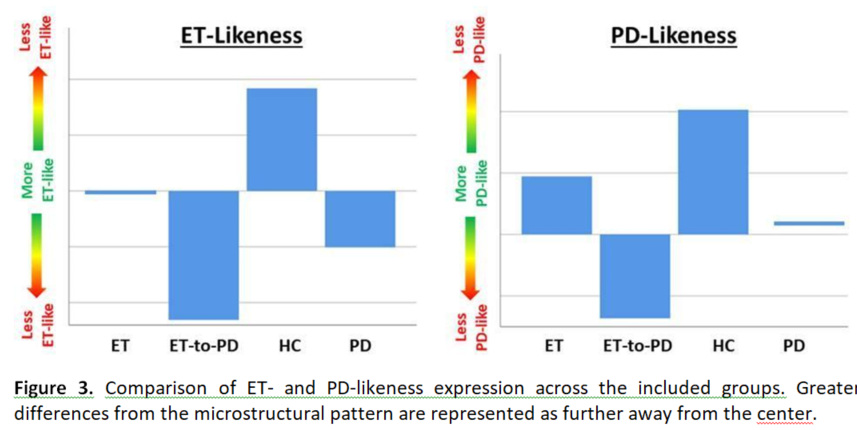
Figure 2 shows the extracted disease-specific tremor-network microstructure PCA-patterns. Figure 3 compares the groups on the PCA-pattern scores, (one-way ANOVA: p=0.011, p=0.031 for ET/PD, respectively), including an ET-to-PD conversion sub-group.
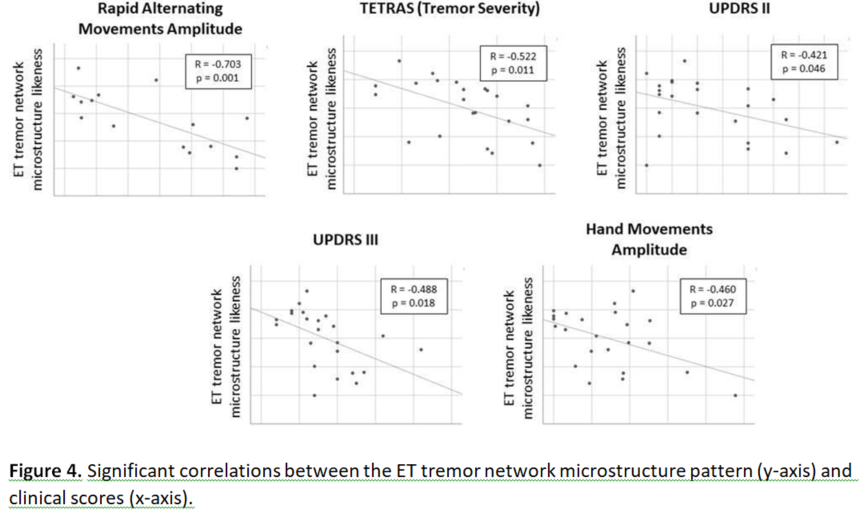
In the ET group, the tremor-network PCA-score was significantly correlated (after multiple comparison correction) with age (p=0.011), TETRAS (p=0.005), UPDRS-III (p=0.001) and motion-sensor measures: “rapid-alternating-movements-amplitude” (p<0.001), “rapid-alternating-movements- rhythm” (p=0.022) and “hand-movement-amplitude” (p=0.025). In the PD group, the tremor-network score was not significantly correlated with any variables (Figure 4).
Conclusion: We present the first study of DSI in ET. Differentiation of ET and PD by DSI is supported by the non-overlap of significant differences in the region-wise analysis, and by variation in the expression of ET and PD tremor-network likeness. MRI-based markers may aid clinical differential diagnosis of ET and PD.
References: 1. Welton T, Chan L, Carr J, Cardoso F, Deutchl G, Jankovic J & Tan EK, 2021. Essential Tremor. Nature Reviews Disease Primers. 7(1): 83.
2. Wedeen VJ, Hagmann P, Tseng WYI, Reese TG and Weisskoff RM, 2005. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magnetic Resonance in Medicine. 1377-1386.
3. Giuffrida JP, Riley DE, Maddux BN & Heldman DA, 2009. Clinically deployable Kinesia technology for automated tremor assessment. Movement Disorders. 24(5):723-30.
4. Yeh FC, 2021. DSI Studio (Version 2021 May). Zenodo. http://doi.org/10.5281/zenodo.4764264
To cite this abstract in AMA style:
T. Welton, A. Ong, S. Hartono, Y-C. Shih, A. Lee, E-K. Tan, L-L. Chan. Distinct Patterns of Tremor Network Microstructure for Essential Tremor and Parkinson’s Disease [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/distinct-patterns-of-tremor-network-microstructure-for-essential-tremor-and-parkinsons-disease/. Accessed April 26, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/distinct-patterns-of-tremor-network-microstructure-for-essential-tremor-and-parkinsons-disease/